- AI
- Molecular Imaging
- CT
- X-Ray
- Ultrasound
- MRI
- Facility Management
- Mammography
FDA Clears Cardiac and Lung AI Applications for Exo Iris Handheld Ultrasound
The artificial intelligence (AI)-powered applications reportedly allow clinicians to diagnose pulmonary edema and measure left ventricle ejection fraction within seconds.
The Food and Drug Administration (FDA) has granted 510(k) clearance for the addition of cardiac and lung artificial intelligence (AI) applications for the Exo Iris handheld ultrasound device.
With rapid identification of B-lines, the lung AI application allows quick detection of pulmonary edema, according to Exo. Whether one is utilizing parasternal long axis (PLAX) or apical four-chamber views, Exo said the cardiac AI application enables clinicians to ascertain stroke volume and measure left ventricle ejection fraction (LVEF) in seconds.
Newly FDA-cleared cardio and lung AI applications for the Exo Iris handheld ultrasound device may facilitate assessments of left ventricle ejection fraction (LVEF) and pulmonary edema in seconds, according to Exo, the manufacturer of the Exo Iris device. (Images courtesy of Exo.)
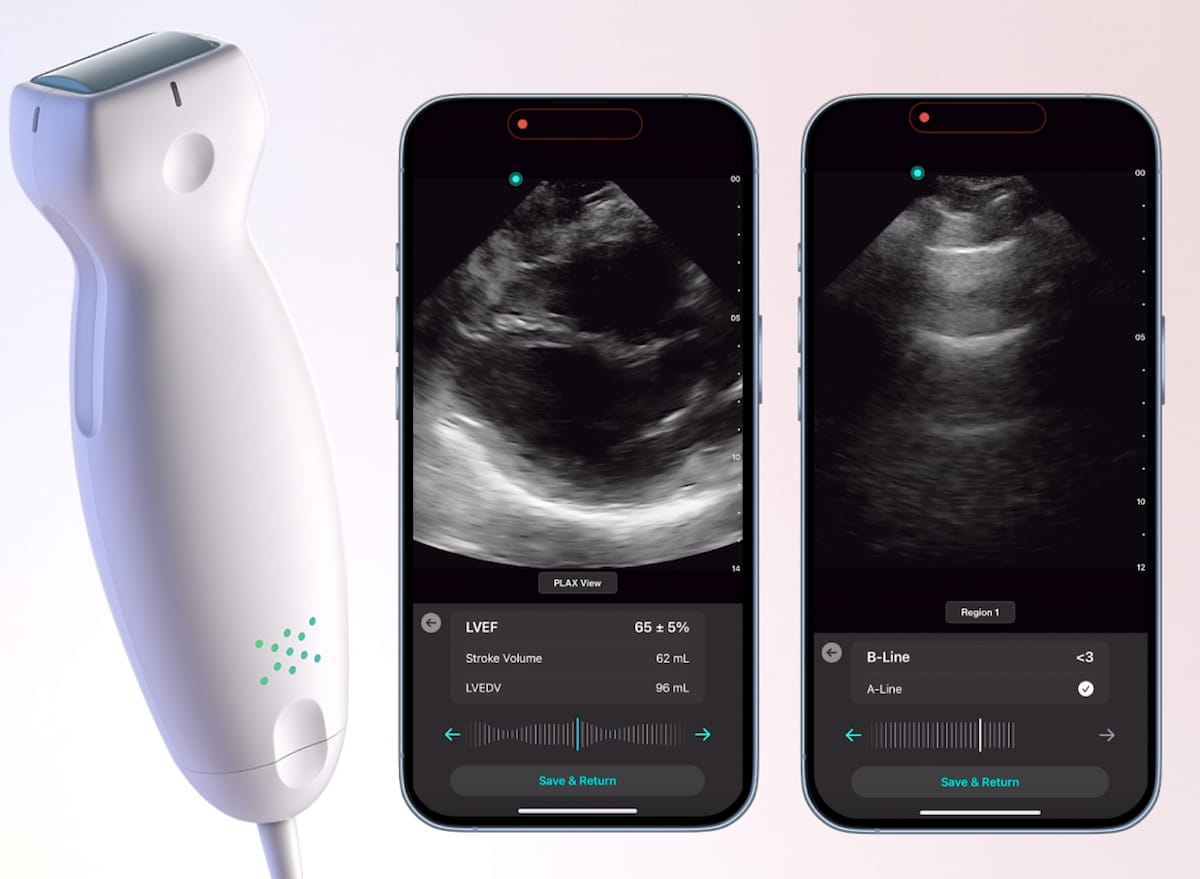
Emphasizing that the AI applications have been trained over 100,000 ultrasound images, including point-of-care images taken in critical care units and emergency room settings, Exo maintained that the cardiac and lung AI applications provide real-time recognition of key imaging landmarks, even with less than perfect scans.
“Exo’s cardiac and lung AI applications are a game-changer,” said Ted Koutouzis, M.D., a clinical instructor in emergency medicine at Northwestern Medicine in Chicago. “Now I have a fast and reliable clinical tool to help easily distinguish between COPD and CHF patients for precise and timely care.”
New Literature Review Finds ChatGPT Effective in Radiology in 84 Percent of Studies
April 29th 2024While noting a variety of pitfalls with the chatbot ranging from hallucinations to improper citations, the review authors found the use of ChatGPT in radiology demonstrated “high performance” in 37 out of 44 studies.
European Society of Breast Imaging Issues Updated Breast Cancer Screening Recommendations
April 24th 2024One of the recommendations from the European Society of Breast Imaging (EUSOBI) is annual breast MRI exams starting at 25 years of age for women deemed to be at high risk for breast cancer.
The Reading Room: Artificial Intelligence: What RSNA 2020 Offered, and What 2021 Could Bring
December 5th 2020Nina Kottler, M.D., chief medical officer of AI at Radiology Partners, discusses, during RSNA 2020, what new developments the annual meeting provided about these technologies, sessions to access, and what to expect in the coming year.
Could a Deep Learning Model for Mammography Improve Prediction of DCIS and Invasive Breast Cancer?
April 15th 2024Artificial intelligence (AI) assessment of mammography images may significantly enhance the prediction of invasive breast cancer and ductal carcinoma in situ (DCIS) in women with breast cancer, according to new research presented at the Society for Breast Imaging (SBI) conference.