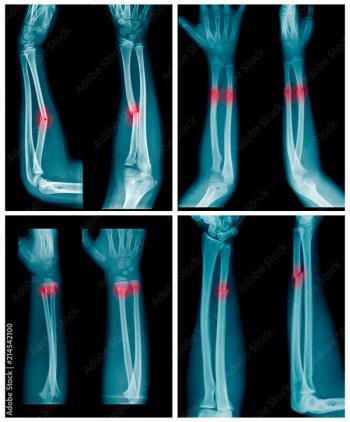
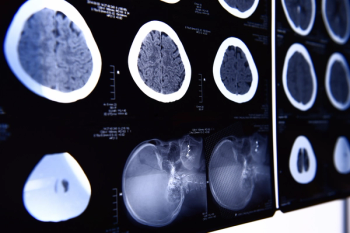
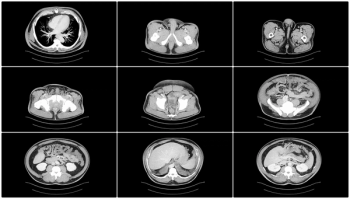
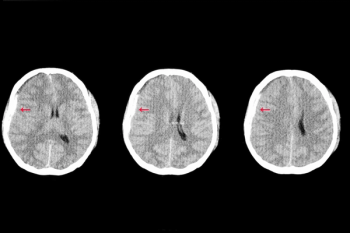
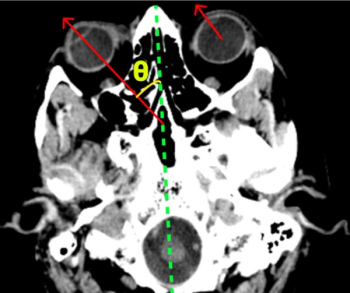
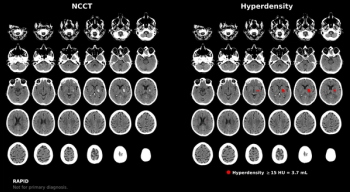
Offering more than 70 clinical applications, the next-generation edition of the Advanced Visualization Workspace reportedly includes enhanced liver analysis and artificial intelligence (AI)-powered scoring of early brain infarction noted on computed tomography (CT) scans for patients with ischemic stroke.