Emerging PET Agent Garners Second FDA Fast Track Designation for Prostate Cancer Imaging
In addition to an August FDA fast track designation for PSMA PET imaging in patients with suspected metastasis, the radiopharmaceutical 64Cu-SAR-bisPSMA has earned another fast track designation for imaging of biochemical recurrence.
The prostate-specific membrane antigen positron emission tomography (PSMA PET) agent 64Cu-SAR-bisPSMA has now been granted a second fast track designation by the Food and Drug Administration (FDA) for prostate cancer (PCa) imaging.
The PET agent’s latest FDA fast track designation, for biochemical PCa recurrence in patients who have had definitive therapy, comes five months after the initial fast track designation for suspected metastasis in patients who are candidates for definitive PCa treatment, according to Clarity Pharmaceuticals, the developer of 64Cu-SAR-bisPSMA.
Here one can see a comparison of the PET agents 64Cu-SAR-bisPSMA and 68Ga PSMA-11 PET/CT. The FDA has granted two fast track designations for 64Cu-SAR-bisPSMA with the latest one being for biochemical prostate cancer recurrence in patients who have had definitive therapy. (Images courtesy of the Society of Nuclear Medicine and Molecular Imaging (SNMMI).)
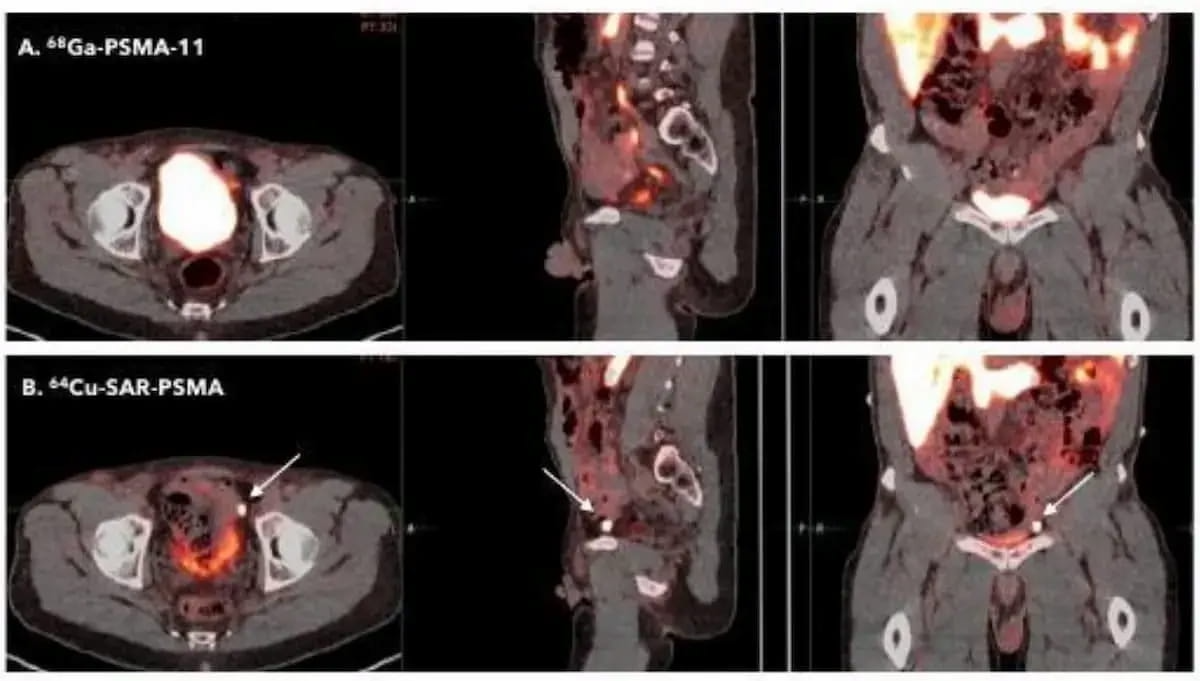
"Receiving the second (fast track designation) for 64Cu-SAR-bisPSMA and well within the 60-day period following our application submission, reserved by the U.S. FDA for review, is yet another significant milestone in our bisPSMA program,” noted Alan Taylor, Ph.D., the executive chairperson of Clarity Pharmaceuticals. “This highlights the high unmet need for novel diagnostics in prostate cancer and the high quality of data we presented to the FDA.”
Emphasizing the increased uptake of 64Cu-SAR-bisPSMA in PCa lesions, Clarity Pharmaceuticals noted that recent research has demonstrated the ability of 64Cu-SAR-bisPSMA to diagnose recurrent lesions in the 2 mm range months before detection by other PSMA PET agents.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.
The Reading Room Podcast: Current Perspectives on the Updated Appropriate Use Criteria for Brain PET
March 18th 2025In a new podcast, Satoshi Minoshima, M.D., Ph.D., and James Williams, Ph.D., share their insights on the recently updated appropriate use criteria for amyloid PET and tau PET in patients with mild cognitive impairment.
New PET Study Links Higher Education Level to Speed of Tau Accumulation with Alzheimer’s Disease
July 8th 2025For patients with amyloid β (Aβ)-positive findings on positron emission tomography, higher educational attainment was associated with accelerated accumulation and spread of tau, according to new research.