FDA Clears AI Software for Assessing Intracerebral Hemorrhage on Non-Contrast CT
Facilitating timely diagnosis and intervention for brain bleeds, the artificial intelligence (AI)-powered Viz ICH Plus software may provide quantified assessment for intracranial hyperdensities, lateral ventricles and midline shifts.
The Food and Drug Administration (FDA) has granted 510(k) clearance for Viz ICH Plus software, which utilizes artificial intelligence (AI) to help quantify intracerebral hemorrhage on non-contrast computed tomography (CT) images.
For cases involving intracerebral hemorrhage, Viz ICH Plus offers automated detection, labeling, and volume quantification for segmentable brain structures, according to Viz.ai, the developer of the software.
The newly FDA-cleared Viz ICH Plus reportedly offers automated detection, labeling and volume quantification of intracerebral hemorrhage on non-contrast CT. (Image courtesy of Viz.ai.)
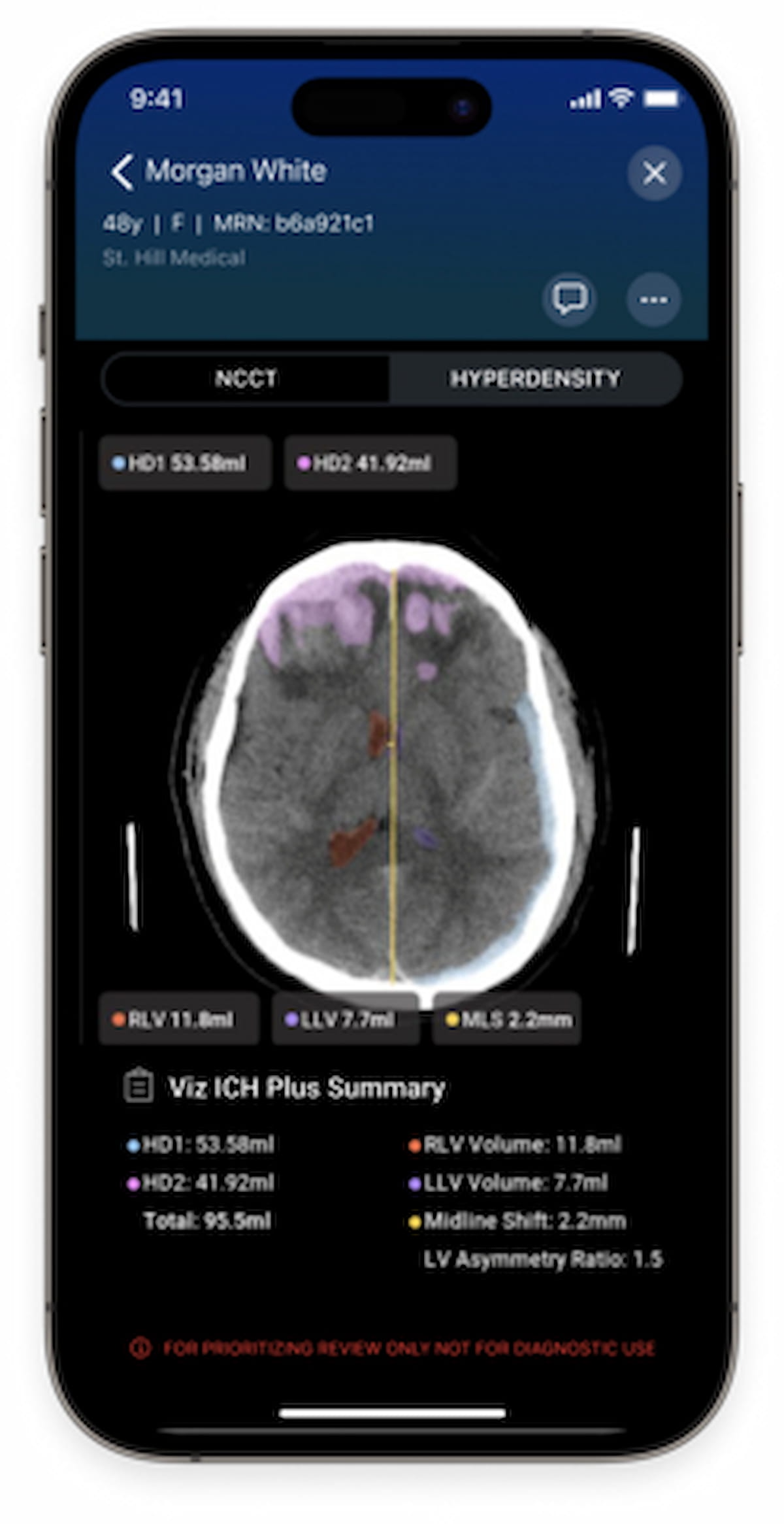
Given the critical nature of intracerebral hemorrhage, which reportedly accounts for 15 percent of strokes, the capability of Viz ICH Plus software to analyze intracranial hyperdensities, lateral ventricles and midline shifts may improve timely diagnosis and decision-making for appropriate treatments.
"The ability and mobility to obtain accurate and quantifiable measurements of intracerebral hemorrhages through Viz ICH Plus significantly enhances our decision-making process,” said Peter Kan, MD, MPH, FRCSC, FAANS, a professor and chair of the Department of Neurosurgery at the University of Texas Medical Branch. “This technology, which marries precision with AI, is poised to transform how we approach intracerebral hemorrhage cases."
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.
Stroke MRI Study Assesses Impact of Motion Artifacts Upon AI and Radiologist Lesion Detection
July 16th 2025Noting a 7.4 percent incidence of motion artifacts on brain MRI scans for suspected stroke patients, the authors of a new study found that motion artifacts can reduce radiologist and AI accuracy for detecting hemorrhagic lesions.