FDA Clears Automated Dosimetry with the Monte Carlo Method for Radionuclide Therapy
The clearance may facilitate improved standardization and efficiency of dosimetry-guided, theranostic applications of radiopharmaceutical therapy.
While calculations with previous use of Monte Carlo dosimetry could take hours, the Food and Drug Administration (FDA) has granted 510(k) clearance for a dose planning method that facilitates automated use of this dosimetry technique in seconds for radiotherapy applications.
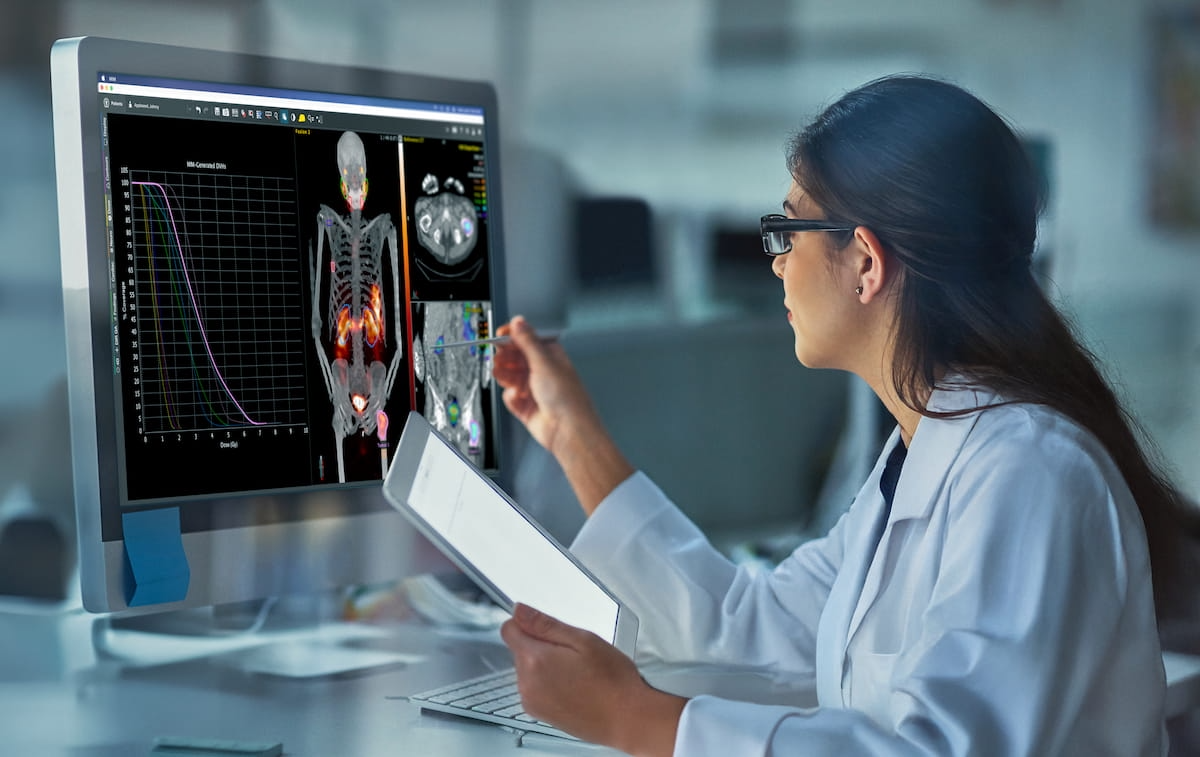
Clinicians will now be able to access automated Monte Carlo dosimetry with the dose planning method through the MIM SurePlan MRT platform (MIM Software/GE HealthCare). While GE HealthCare said the Monte Carlo method has been acknowledged as a gold standard in dosimetry for radiophamaceutical therapy, clinicians can now review absorbed doses with this technique in a few clicks.
The availability of a dose planning method through the MIM SurePlan MRT platform may allow clinicians access to automated dosimetry assessment with the Monte Carlo method in seconds, according to GE HealthCare, the manufacturer of the MIM SurePlan MRT platform. (Photo courtesy of GE HealthCare.)
“It is exciting that the dose planning method code developed and validated at our institution will be available to the theranostics community at large with the recent FDA clearance,” said Yuni Dewaraja, PhD, a professor of radiology at the University of Michigan. “This opens up the possibility for harmonized and accurate patient-specific dosimetry across centers, which can lead to robust dose-effect relationships, dosimetry-guided radiopharmaceutical therapy, and ultimately a greater benefit for patients receiving these promising therapies.”
GE HealthCare added that the MIM SurePlan MRT automates dosimetry processes such as organ-at-risk segmentation, time-activity curve fitting and single photon emission computed tomography (SPECT) reconstruction.
The Reading Room Podcast: Current Perspectives on the Updated Appropriate Use Criteria for Brain PET
March 18th 2025In a new podcast, Satoshi Minoshima, M.D., Ph.D., and James Williams, Ph.D., share their insights on the recently updated appropriate use criteria for amyloid PET and tau PET in patients with mild cognitive impairment.
FDA Grants Fast Track Designation to Emerging Agent for Brain PET Imaging
June 11th 2025Currently being evaluated in a phase 2b clinical trial, the 18F-RAD101 PET imaging agent garnered the FDA’s fast track designation for distinguishing between recurrent disease and treatment impact for brain metastases derived from solid tumors.
New PSMA PET Prep Product Now Available in the U.S.
June 11th 2025Offering an extended shelf life, the FDA-approved Gozellix, a preparation kit for gallium-68 (68Ga) gozetotide injection, is indicated for use in PSMA PET imaging of prostate cancer patients with suspected recurrence or metastasis.