FDA Clears New Version of AI Segmentation Software for Brain MRI
Offering rapid automated segmentation of brain structures, the ClearPoint 2.2 software reportedly enhances visualization for a variety of targeted, MRI-guided procedures.
The Food and Drug Administration (FDA) has granted 510(k) clearance for the artificial intelligence (AI)-enabled ClearPoint 2.2 software, which provides automated segmentation of brain structures from magnetic resonance imaging (MRI) scans.
ClearPoint Neuro, the manufacturer of the software, said it provides rapid segmentation that facilitates a variety of targeted, MRI-guided procedures ranging from deep brain stimulation to laser ablation.
Here one can see a comparison of the ClearPoint Maestro Brain Model (a) and the ClearPoint 2.2 software (b), which includes segmentation of subnuclei. (Images courtesy of NeuroImage.)
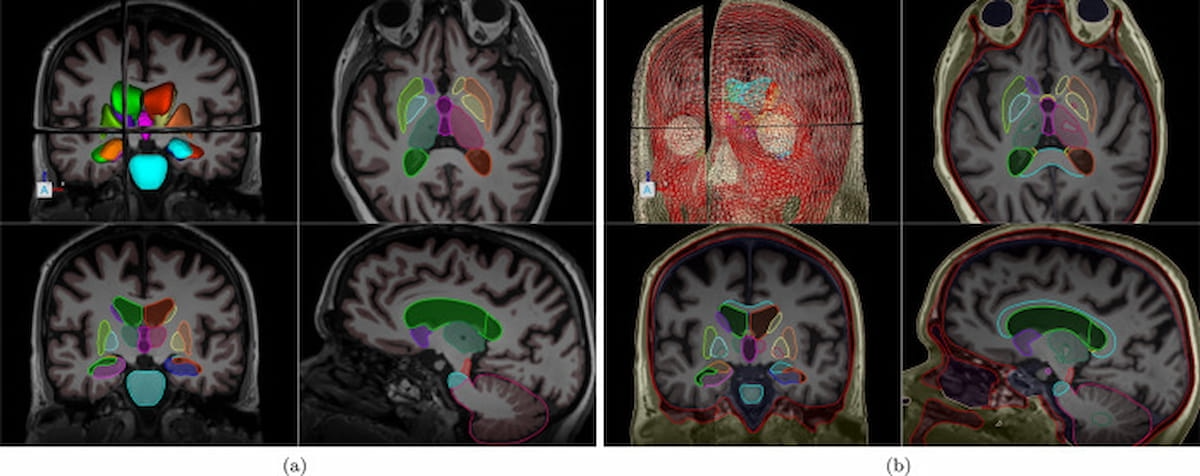
The ClearPoint 2.2 software also includes the Maestro Brain Model, which was recently validated in a study published in NeuroImage. In a quantitative reproducibility analysis, researchers found the combination of the shape-constrained deformable brain model and voxel-wise tissue segmentation was superior to manual expert segmentation.
“The ClearPoint system can now offer fast, peri-procedural segmentation of the cortical structures of the brain to identify both targets and safety zones for cell and gene therapy delivery, laser ablation, biopsy and deep brain stimulation,” noted Joe Burnett, the president and CEO of ClearPoint Neuro.
Considering Breast- and Lesion-Level Assessments with Mammography AI: What New Research Reveals
June 27th 2025While there was a decline of AUC for mammography AI software from breast-level assessments to lesion-level evaluation, the authors of a new study, involving 1,200 women, found that AI offered over a seven percent higher AUC for lesion-level interpretation in comparison to unassisted expert readers.
FDA Clears Virtually Helium-Free 1.5T MRI System from Siemens Healthineers
June 26th 2025Offering a cost- and resource-saving DryCool magnet technology, the Magnetom Flow.Ace MRI system reportedly requires 0.7 liters of liquid helium for cooling over the lifetime of the device in contrast to over 1,000 liters commonly utilized with conventional MRI platforms.