FDA Clears PET-Based Software for Centiloid Scaling of Amyloid Plaque Density
Now available with MIMneuro, the Centiloid scaling software provides automated quantitative assessment of amyloid plaque density, a key component of Alzheimer’s disease.
The Food and Drug Administration (FDA) has granted 510(k) clearance to new Centiloid scaling software that enhances the assessment of amyloid plaque density on positron emission tomography (PET) and may facilitate the use of amyloid-targeted therapies for patients with Alzheimer’s disease.
Through the assessment of PET amyloid imaging, the Centiloid scaling software reportedly provides automated quantitative information on amyloid plaque density, according to MIM Software/GE HealthCare, the developer of the software. The company emphasized that amyloid plaque density is a key component of Alzheimer’s disease.
Through the assessment of PET amyloid imaging, the newly FDA-cleared Centiloid scaling software reportedly provides automated quantitative information on amyloid plaque density, according to MIM Software/GE HealthCare, the developer of the software. (Image courtesy of MIM Software/GE HealthCare.)
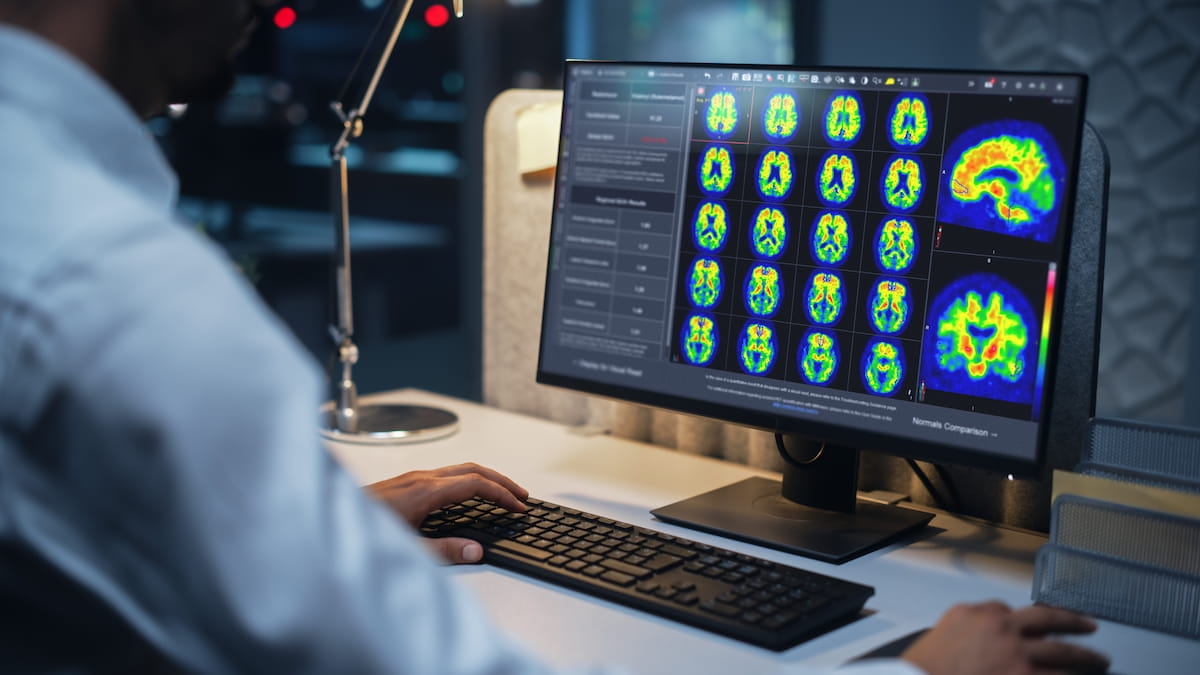
MIM Software said the Centiloid scaling software, now available with MIMneuro, also facilitates workflow efficiency by providing the quantitative assessments of amyloid plaque density alongside the PET images in a standardized report.
“Alzheimer’s is a far-reaching disease that has been a challenge for our society, patients, caregivers, and healthcare systems for decades,” explains Andrew Nelson, CEO of MIM Software, GE HealthCare. “Centiloid scaling with MIMneuro offers a standardized, quantitative metric to assist healthcare providers in confidently estimating amyloid plaque density, one key aspect of this debilitating disease. By increasing clinician confidence, we hope to ultimately expand patient access to cutting-edge, personalized care.”
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.
The Reading Room Podcast: Current Perspectives on the Updated Appropriate Use Criteria for Brain PET
March 18th 2025In a new podcast, Satoshi Minoshima, M.D., Ph.D., and James Williams, Ph.D., share their insights on the recently updated appropriate use criteria for amyloid PET and tau PET in patients with mild cognitive impairment.