FDA Clears CT-Based AI Software for Enhanced Detection of Usual Interstitial Pneumonia
The IQ-UIP AI software may bolster computed tomography diagnosis of usual interstitial pneumonia (UIP), which is reportedly misdiagnosed in over 50 percent of cases.
The Food and Drug Administration (FDA) has granted 510(k) clearance for IQ-UIP, an artificial intelligence (AI)-powered software for enhancing computed tomography (CT) diagnosis of usual interstitial pneumonia (UIP).
Leveraging deep learning technology, IQ-UIP provides automated analysis of conventional CT scans with rapid detection of UIP patterns such as honeycombing and subpleural fibrosis, according to 4DMedical, the developer of IQ-UIP.
In addition to facilitating improved detection of usual interstitial pneumonia (UIP) on standard CT scans, 4DMedical says the newly FDA-cleared AI software IQ-UIP provides automated referral workflows for specialists who treat UIP. (Image courtesy of RadiologyinThai.com )
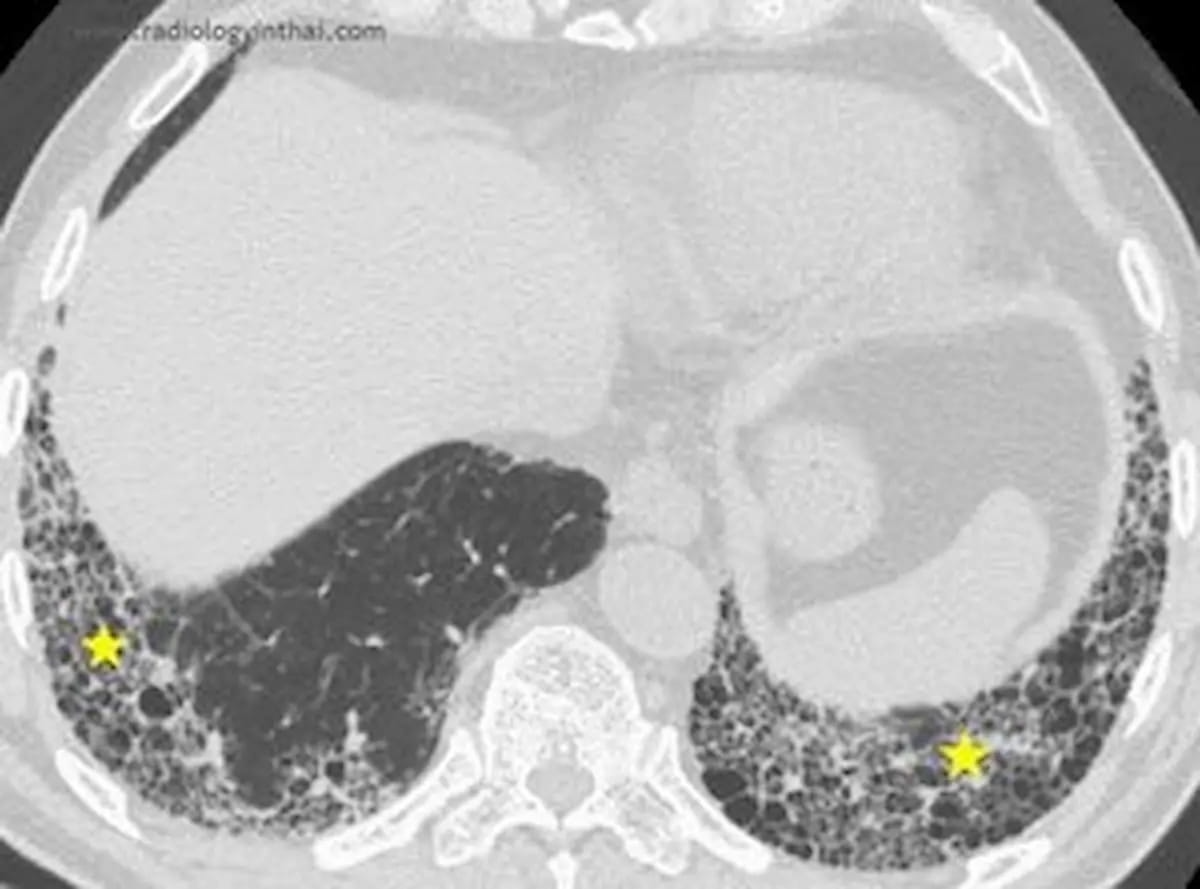
Emphasizing that more than 50 percent of UIP is initially misdiagnosed, 4DMedical noted the condition’s non-specific symptomatology may mimic other conditions such as asthma and chronic obstructive pulmonary disease (COPD).
In addition to a rapid diagnosis of UIP, which is characterized by chronic inflammation and progressive lung fibrosis, 4DMedical said IQ-UIP provides automated referral workflows for specialists, facilitating timely care for a condition that has a median survival of one to two years after diagnosis.
Considering Breast- and Lesion-Level Assessments with Mammography AI: What New Research Reveals
June 27th 2025While there was a decline of AUC for mammography AI software from breast-level assessments to lesion-level evaluation, the authors of a new study, involving 1,200 women, found that AI offered over a seven percent higher AUC for lesion-level interpretation in comparison to unassisted expert readers.
Can CT-Based Deep Learning Bolster Prognostic Assessments of Ground-Glass Nodules?
June 19th 2025Emerging research shows that a multiple time-series deep learning model assessment of CT images provides 20 percent higher sensitivity than a delta radiomic model and 56 percent higher sensitivity than a clinical model for prognostic evaluation of ground-glass nodules.
FDA Clears Ultrasound AI Detection for Pleural Effusion and Consolidation
June 18th 2025The 14th FDA-cleared AI software embedded in the Exo Iris ultrasound device reportedly enables automated detection of key pulmonary findings that may facilitate detection of pneumonia and tuberculosis in seconds.