FDA Clears Dual-Head SPECT/CT System with Deep Learning Image Reconstruction
The combination of the Aurora SPECT/CT system with AI-enabled Clarify DL image reconstruction reportedly offers the potential of enhanced image quality and streamlined workflows in nuclear medicine.
The Food and Drug Administration (FDA) has granted 510(k) clearance for the Aurora single photon emission computed tomography/computed tomography (SPECT/CT) system and Clarify DL deep learning image reconstruction.
GE HealthCare, the developer of the dual-head Aurora SPECT/CT system, said the device offers the following key benefits:
The newly FDA-cleared dual-head Aurora SPECT/CT system has a 40 mm detector, which offers twice the coverage of CTs from other hybrid systems, and 128-slice capabilities across imaging applications in neurology, cardiology, and oncology, according to GE HealthCare, the developer of the Aurora SPECT/CT platform. (Image courtesy of GE HealthCare.)
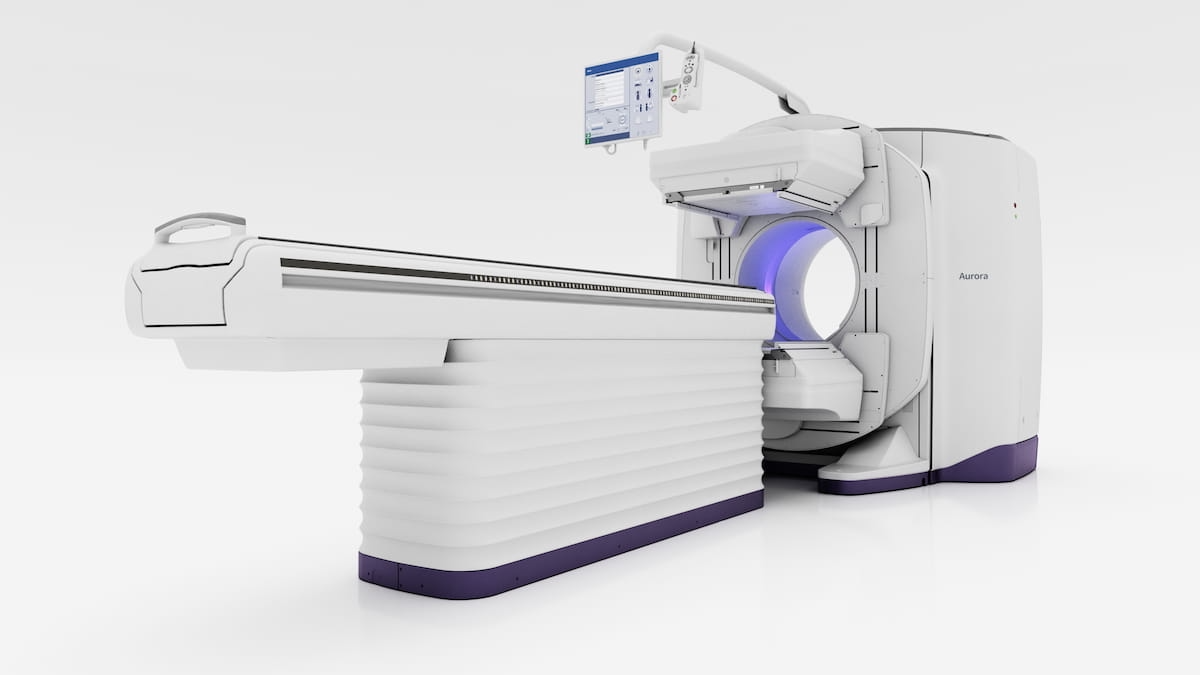
• a 40 mm detector offering twice the coverage of CTs from other hybrid systems;
• 128-slice capabilities across imaging applications in neurology, cardiology, and oncology; and
• a 5/8-inch crystal Nal detector, which allows use of a variety of radiopharmaceuticals including theranostics.
Through advanced deep learning technology, GE HealthCare maintained that Clarity DL image reconstruction bolsters quality for bone SPECT imaging without the need for increased radiation dosing or scan time.
“Aurora’s seamless integration of SPECT and CT components will allow us to perform comprehensive, high-quality diagnostic exams in a single session, while its support of Clarify DL deep-learning image reconstruction enables enhanced image quality performance,” noted Donna Plecha, M.D., chair of the Department of Radiology at University Hospitals Cleveland Medical Center, and a professor of radiology at the Case Western Reserve University School of Medicine in Cleveland.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.
Study Shows Enhanced Diagnosis of Coronary Artery Stenosis with Photon-Counting CTA
July 10th 2025In a new study comparing standard resolution and ultra-high resolution modes for patients undergoing coronary CTA with photon-counting detector CT, researchers found that segment-level sensitivity and accuracy rates for diagnosing coronary artery stenosis were consistently > 89.6 percent.