FDA Grants Fast Track Designation to 64Cu-SAR-bisPSMA PET Agent for Prostate Cancer
Currently in phase III trials, the 64Cu-SAR-bisPSMA PET agent reportedly offers a longer half-life and higher tumor uptake than other radiopharmaceuticals for prostate cancer imaging.
The emerging positron emission tomography (PET) agent 64Cu-SAR-bisPSMA has garnered a fast track designation status from the Food and Drug Administration (FDA).
Separate phase I trials have demonstrated the safety and efficacy of 64Cu-SAR-bisPSMA for the imaging of prostate cancer (PCa) prior to radical prostatectomy and in patients with biochemical recurrence of PCa, according to Clarity Pharmaceuticals, the developer of the PET agent.
Recent research suggested that 64Cu-SAR-bisPSMA has a higher detection rate than 68Ga PSMA-11 PET/CT for prostate cancer. Currently in phase III trials, the 64Cu-SAR-bisPSMA PET agent recently garnered fast track designation status from the Food and Drug Administration (FDA). (Images courtesy of the Society of Nuclear Medicine and Molecular Imaging (SNMMI)).
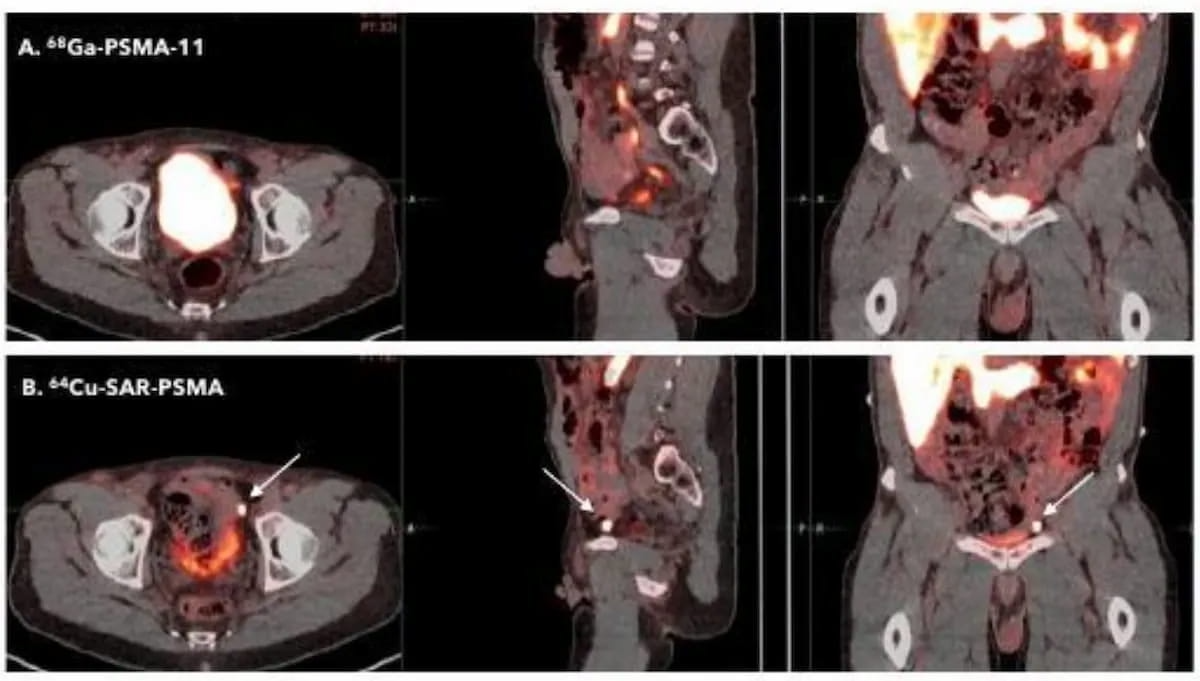
In comparative research presented at the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) conference, researchers found that 64Cu-SAR-bisPSMA had a higher detection rate for PCa and a higher median tumor-to-background ratio (TBR) in comparison to 68Ga PSMA-11 PET/CT. Clarity Pharmaceuticals noted that current phase III trials are ongoing for 64Cu-SAR-bisPSMA.
"We believe that 64Cu-SAR-bisPSMA could be a game changer in prostate cancer diagnosis. Due to its dual targeting structure, bisPSMA, and the longer half-life of copper-64, enabling next-day imaging, this unique product has shown higher tumor uptake and retention and exhibited a capability of detecting much smaller lesions,” noted Alan Taylor, the executive chairperson for Clarity Pharmaceuticals.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.
Multinational Study Reaffirms Value of Adjunctive AI for Prostate MRI
June 16th 2025The use of adjunctive AI in biparametric prostate MRI exams led to 3.3 percent and 3.4 percent increases in the AUC and specificity, respectively, for clinically significant prostate cancer (csPCa) in a 360-person cohort drawn from 53 facilities.