Emerging PET Imaging Agent Gets FDA’s Breakthrough Therapy Designation for Cardiac Amyloidosis
Reportedly the only imaging agent to garner a breakthrough therapy designation for cardiac amyloidosis, 124I-evuzamitide has demonstrated robust sensitivity for the condition as well as a favorable safety profile.
The Food and Drug Administration (FDA) has granted a breakthrough therapy designation for use of the positron emission tomography (PET) imaging agent 124I-evuzamitide (AT-01) for patients with suspected or known cardiac amyloidosis.
The first non-invasive pan-amyloid PET agent designed for systemic amyloidosis, 124I-evuzamitide has reportedly demonstrated effectiveness in detecting multiple types of amyloid deposits in the liver, kidney, and heart, according to Attralus, the developer of 124I-evuzamitide.1
The first non-invasive pan-amyloid PET agent designed for systemic amyloidosis, 124I-evuzamitide recently garnered a breakthrough therapy designation from the FDA for use in patients with suspected or known cardiac amyloidosis. (Images courtesy of Attralus.)
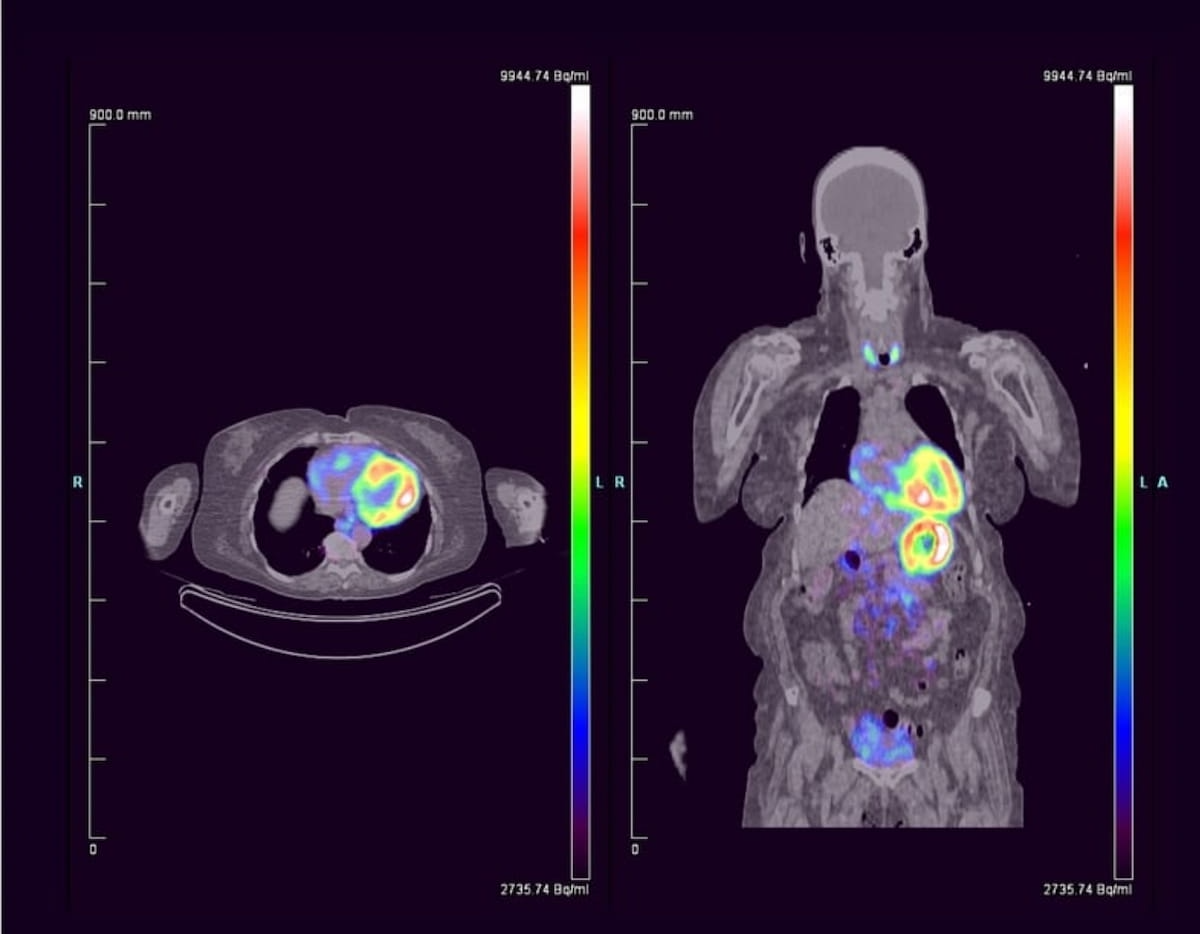
In a recent study evaluating the use of 124I-evuzamitide for 50 patients with systemic amyloidosis, researchers noted a 96.2 percent cardiac-associated sensitivity rate and one drug-related adverse event.2
Emphasizing that 124I-evuzamitide is the only diagnostic imaging agent to garner a breakthrough therapy designation for cardiac amyloidosis, Attralus says the FDA’s decision was based on multiple studies evaluating use of the radiotracer in patients with cardiac amyloidosis.1
“We are highly encouraged by FDA’s decision to grant Breakthrough Therapy Designation to 124I-evuzamitide (AT-01), recognizing its potential as an innovative diagnostic agent for patients with systemic amyloidosis” said Gregory Bell, M.D., the chief medical officer at Attralus. “There are no FDA approved diagnostic imaging agents for cardiac amyloidosis. The diagnosis of cardiac amyloidosis is a challenging and time-consuming process for patients, with many going years without an accurate diagnosis, and losing critical time in the process.”
Attralus added that 124I-evuzamitide (AT-01) will be the subject of a phase 3 study of patients with suspected cardiac amyloidosis that is slated to begin in 2025.
References
1. Attralus. Attralus receives breakthrough therapy designation for its pan-amyloid diagnostic PET imaging candidate 124I-evuzamitide (AT-01) for cardiac amyloidosis. Globe Newswire. Available at: https://www.globenewswire.com/news-release/2024/08/05/2924179/0/en/Attralus-Receives-Breakthrough-Therapy-Designation-for-its-Pan-Amyloid-Diagnostic-PET-Imaging-Candidate-124I-evuzamitide-AT-01-for-Cardiac-Amyloidosis.html . Published August 5, 2024. Accessed August 5, 2024.
2. Wall JS, Martin EB, Lands R, et al. Cardiac amyloid detection by PET/CT imaging of iodine (124I) evuzamitide (124I-p5+14): a phase 1/2 study. JACC Cardiovasc Imaging. 2023;16(11):1433-1448.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.
The Reading Room Podcast: Current Perspectives on the Updated Appropriate Use Criteria for Brain PET
March 18th 2025In a new podcast, Satoshi Minoshima, M.D., Ph.D., and James Williams, Ph.D., share their insights on the recently updated appropriate use criteria for amyloid PET and tau PET in patients with mild cognitive impairment.
New PET Study Links Higher Education Level to Speed of Tau Accumulation with Alzheimer’s Disease
July 8th 2025For patients with amyloid β (Aβ)-positive findings on positron emission tomography, higher educational attainment was associated with accelerated accumulation and spread of tau, according to new research.