SNMMI: New Study Suggests Merits of FAPI PET/CT for Breast Cancer Staging
Improved assessment of axillary lymph node status with FAPI PET/CT led to restaging of nearly 71 percent of patients with breast cancer, according to new research presented at the SNMMI 2024 conference.
Can fibroblast activation protein inhibitor (FAPI) PET/CT have an impact in staging for women with newly diagnosed breast cancer?
For the study, which was presented at the 2024 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, researchers compared clinical TNM staging between FAPI PET/CT and 18F-FDG PET/CT, which is commonly utilized in breast cancer staging. The cohort was comprised of 121 women with breast cancer. According to the study, 53 women had 68Ga-FAPI-04 PET/CT, 68 study participants had A118F-FAPI-04 PET/CT and all patients had 18F-FDG PET/CT scans.
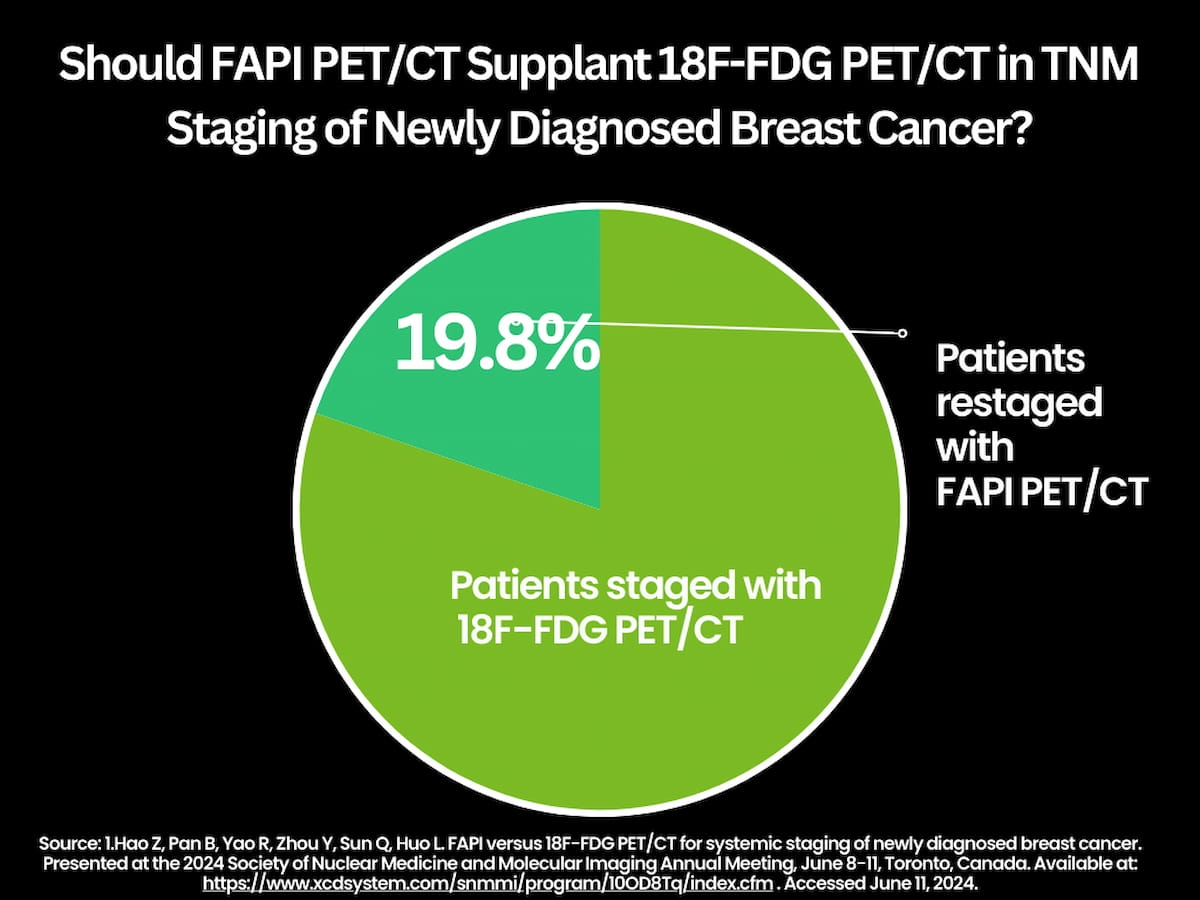
The researchers found that FAPI PET/CT, in comparison to TNM staging with 18F-FDG PET/CT, restaged 19.8 percent of patients (24/121). Assessment of axillary lymph node (ALN) status by FAPI PET/CT was a significant factor for 17 of 24 patients (70.8 percent) who had TNM restaging, according to the study authors.
New research presented at the SNMMI 2024 conference found that FAPI PET/CT, in comparison to TNM staging with 18F-FDG PET/CT, restaged 19.8 percent of patients (24/121) with newly diagnosed breast cancer,
Noting the challenges with 18F-FDG PET/CT with respect to reduced sensitivity in certain breast cancer subtypes and false positives with inflammatory breast lesions, the researchers said the study findings show that FAPI PET/CT can be a viable alternative.
“This study is significant as it has the potential to advance personalized treatment strategies for breast cancer patients,” noted lead study author Zhixin Hao, M.D., a nuclear medicine physician affiliated with Peking Union Medical College Hospital in Beijing, China. “FAPI PET/CT for the initial staging of breast cancer has the potential to reduce unnecessary treatments and improve patient outcomes.”
The researchers also pointed out that FAPI PET/CT down-staged 20.6 percent of women (7/34) with initial IIB staging andupstaged 21.7 percent of patients (10/46) with initial IIA staging.
“Patients with stage IIA (breast cancer) should be considered for systemic staging with FAPI PET/CT at the time of initial diagnosis,” emphasized Hao and colleagues.
Reference
1. Hao Z, Pan B, Yao R, Zhou Y, Sun Q, Huo L. FAPI versus 18F-FDG PET/CT for systemic staging of newly diagnosed breast cancer. Presented at the 2024 Society of Nuclear Medicine and Molecular Imaging Annual Meeting, June 8-11, Toronto, Canada. Available at: https://www.xcdsystem.com/snmmi/program/10OD8Tq/index.cfm . Accessed June 11, 2024.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.
The Reading Room Podcast: Current Perspectives on the Updated Appropriate Use Criteria for Brain PET
March 18th 2025In a new podcast, Satoshi Minoshima, M.D., Ph.D., and James Williams, Ph.D., share their insights on the recently updated appropriate use criteria for amyloid PET and tau PET in patients with mild cognitive impairment.
New PET Study Links Higher Education Level to Speed of Tau Accumulation with Alzheimer’s Disease
July 8th 2025For patients with amyloid β (Aβ)-positive findings on positron emission tomography, higher educational attainment was associated with accelerated accumulation and spread of tau, according to new research.
SNMMI: Botox May Facilitate Relief from Dry Mouth Side Effect of PSMA-Targeted Radiopharmaceuticals
June 25th 2025For patients being treated with radiopharmaceutical agents for metastatic prostate cancer, the combination of botulinum toxin and an anti-nausea patch led to a 30 percent reduction in PSMA uptake in the salivary glands, according to preliminary research findings presented at the SNMMI conference.
SNMMI: Can 18F-Fluciclovine PET/CT Bolster Detection of PCa Recurrence in the Prostate Bed?
June 24th 2025In an ongoing prospective study of patients with biochemical recurrence of PCa and an initial negative PSMA PET/CT, preliminary findings revealed positive 18F-fluciclovine PET/CT scans in over 54 percent of the cohort, according to a recent poster presentation at the SNMMI conference.