SNMMI: PSMA-18F DCFPyL Changes Treatment for PCa Recurrence in Significant Number of Patients with Low PSAs
In a cohort of 85 patients with biomechanical recurrence of prostate cancer and PSA values less than 2 ng/mL, positive findings on PET imaging with PSMA-18F DCFPyL led to treatment changes in the majority of patients who had negative findings on conventional imaging, according to new research presented at the SNMMI conference.
The use of PSMA-18F DCFPyL may be more advantageous than conventional imaging in detecting biomechanical recurrence of prostate cancer in patients with low prostate-specific antigen (PSA) values, according to new research presented at the 2024 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting.
For the retrospective study, researchers evaluated the use of PET PSMA-18F DCFPyL (Pylarify, Lantheus) in 85 patients (mean age of 69) who had occult prostate cancer recurrence and PSA values less than 2 ng/mL. All patients in the cohort had negative computed tomography (CT), magnetic resonance imaging (MRI), ultrasound and positron emission tomography (PET)-choline exams, according to the study authors.
At a six-month follow-up, the researchers found that PET imaging with PSMA-18F DCFPyL led to treatment changes in 75 percent of patients with positive findings (31/41).
Here one can see PET/CT imaging with PSMA-18F DCFPyL that led to modification with a planned radiotherapy treatment for recurrent prostate cancer. (Images courtesy of SNMMI.)
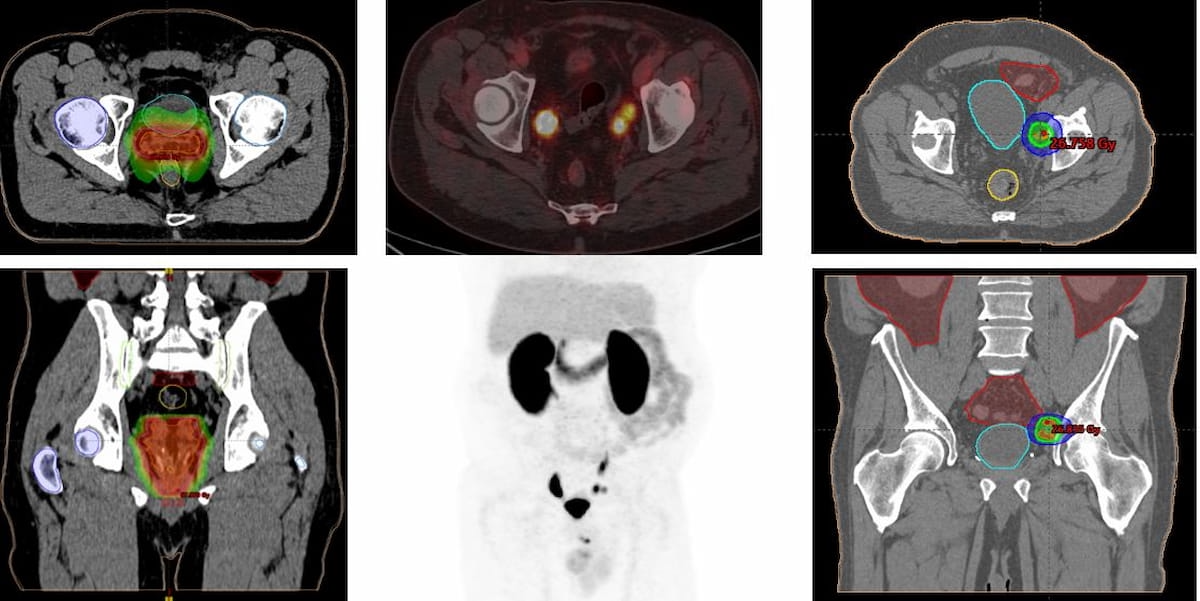
“In PET-PSMA-positive patients, the therapeutic changes found were classified as indicating or changing the planning of salvage radiotherapy treatment and/or initiating systemic treatment with hormone therapy,” noted lead study author Pedro Jose Plana Lopez, M.D., who is affiliated with Hospital Del Mar in Barcelona, Spain, and colleagues.
The study authors noted a median PSA level of 0.08 ng/dL after treatment changes were instituted in patients with positive PET PSMA results.
(Editor’s note: For related content, see “Can an Emerging PET Radiotracer Be a Viable Alternative to Multiparametric MRI for Detecting Prostate Cancer Recurrence?,” “Utilizing AI for Quantitative Assessment of Prostate Cancer Recurrence” and “Emerging PET Radiotracer May Offer Multiple Advantages in Detecting Prostate Cancer.”)
“PET-PSMA is a powerful diagnostic tool for patients with occult biochemical recurrence with PSA < 2ng/mL,” added Lopez and colleagues.
Reference
1. Lopez PJP, Amorelli FG, Cano JSB, et al. PET/CT PSMA-18F DCFPyL promotes treatment changes in occult biochemical recurrence of prostate carcinoma even with low PSA values. Presented at the 2024 Society of Nuclear Medicine and Molecular Imaging Annual Meeting, June 8-11, Toronto, Canada. Available at: https://www.xcdsystem.com/snmmi/program/10OD8Tq/index.cfm . Accessed June 9, 2024.
SNMMI: Botox May Facilitate Relief from Dry Mouth Side Effect of PSMA-Targeted Radiopharmaceuticals
June 25th 2025For patients being treated with radiopharmaceutical agents for metastatic prostate cancer, the combination of botulinum toxin and an anti-nausea patch led to a 30 percent reduction in PSMA uptake in the salivary glands, according to preliminary research findings presented at the SNMMI conference.
The Reading Room Podcast: Current Perspectives on the Updated Appropriate Use Criteria for Brain PET
March 18th 2025In a new podcast, Satoshi Minoshima, M.D., Ph.D., and James Williams, Ph.D., share their insights on the recently updated appropriate use criteria for amyloid PET and tau PET in patients with mild cognitive impairment.
SNMMI: Can 18F-Fluciclovine PET/CT Bolster Detection of PCa Recurrence in the Prostate Bed?
June 24th 2025In an ongoing prospective study of patients with biochemical recurrence of PCa and an initial negative PSMA PET/CT, preliminary findings revealed positive 18F-fluciclovine PET/CT scans in over 54 percent of the cohort, according to a recent poster presentation at the SNMMI conference.
Could an Emerging PET Tracer be a Game Changer for Detecting Hepatocellular Carcinoma?
June 23rd 2025In addition to over 90 percent sensitivity in detecting hepatocellular carcinoma (HCC), the glypican-3 (GPC3) targeted PET tracer 68Ga-aGPC3-scFv appeared to be advantageous in identifying HCC tumors smaller than one centimeter, according to pilot study findings presented at the SNMMI conference.