FDA Clears New Version of Digital Radiography System
Siemens Healthcare announced FDA clearance for its two-detector Multix Fusion system.
The United States Food and Drug Administration (FDA) has provided 510(k) clearance to a new, two-detector version of the digital radiography system, Multix Fusion by Siemens Healthcare.
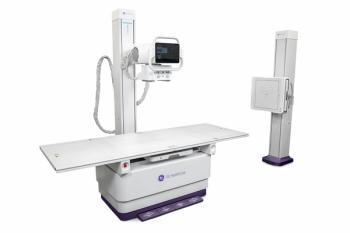
The Multix Fusion was initially introduced in 2012. The newly approved two-detector iteration uses digital cassette-sized flat panel detectors, a large fixed detector in the wall stand, a height-adjustable table with a 660-pound weight capacity, and a touch screen positioned by the overhead tube suspension for viewing exam parameter changes, Siemens announced.[[{"type":"media","view_mode":"media_crop","fid":"31132","attributes":{"alt":"Siemens' Multix Fusion","class":"media-image media-image-right","id":"media_crop_9715349969090","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"3287","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","style":"height: 218px; width: 251px; border-width: 0px; border-style: solid; margin: 1px; float: right;","title":"Siemens' Multix Fusion","typeof":"foaf:Image"}}]]
Siemens declared its Multix Fusion system is offered at an affordable price and is designed for customers with high patient throughput looking for high image quality with low dose. Siemens said the two detectors offer “nearly unlimited” positioning flexibility between the table and the wall stand. A high level of image contrast is a result of its DiamondView Plus on-board post-processing tool, Siemens said, and its automated image processing parameter has varying settings based on organ programs.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.