FDA Approves Fluorescence Imaging System for Detecting Residual Breast Cancer
The combination of the optical imaging agent Lumisight and the fluorescence imaging device Lumicell Direct Visualization System, collectively known as LumiSystem, reportedly offers 84 percent accuracy with real-time detection of residual breast cancer after lumpectomy procedures.
The Food and Drug Administration (FDA) has granted dual approvals for pegulicianine (Lumisight, Lumicell), an optical imaging agent, and Lumicell Direct Visualization System (DVS), a fluorescence imaging device, that may facilitate enhanced detection of residual breast cancer after removal of the primary tumor during lumpectomy procedures.1
Collectively known as the LumiSystem™, Lumisight and Lumicell DVS provide clinicians with real-time visualization that may offer a significant advance over current intraoperative imaging tools employed during lumpectomy procedures, according to Barbara L. Smith, M.D., Ph.D., the director of the Breast Program at Massachusetts General Hospital and a professor of surgery at Harvard Medical School.1
“With LumiSystem, we will now have a technology that is clinically proven to achieve a more complete cancer resection during lumpectomy that could help some patients avoid a second surgery,” noted Dr. Smith.
Dual FDA approvals of the optical imaging agent pegulicianine (Lumisight, Lumicell) and the Lumicell Direct Visualization System (DVS), a fluorescence imaging device, provide a " ... technology that is clinically proven to achieve a more complete cancer resection during lumpectomy that could help some patients avoid a second surgery," noted Barbara L. Smith, M.D., Ph.D., the director of the Breast Program at Massachusetts General Hospital and a professor of surgery at Harvard Medical School. (Image courtesy of Lumicell.)
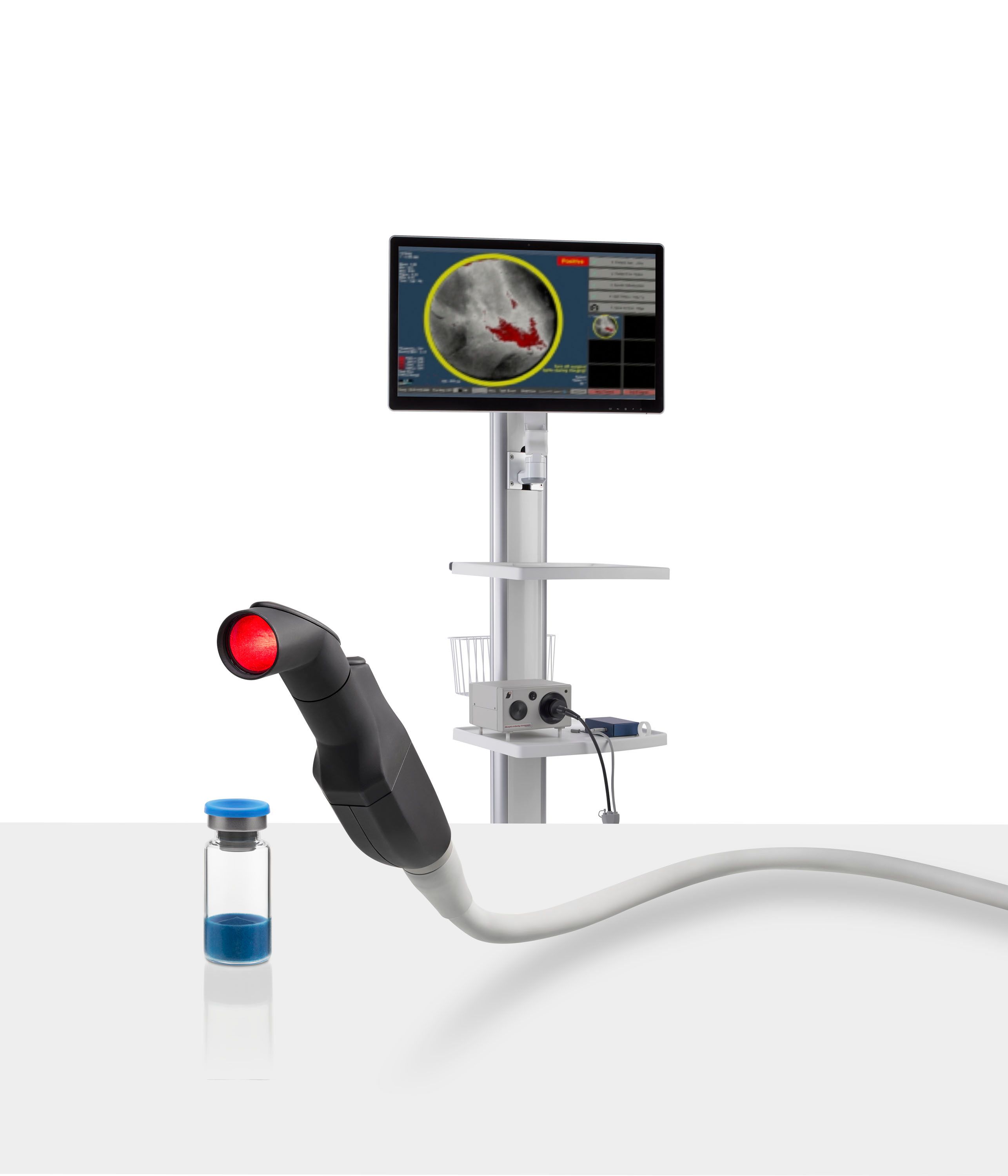
Lumicell said the LumiSystem detects post-lumpectomy residual breast cancer with an 84 percent accuracy rate.1 In a 2023 study published in NEJM Evidence, Smith and colleagues noted that the LumiSystem enabled detection and removal of residual breast cancer after standard lumpectomy in 27 out of 357 patients with 22 of those patients originally deemed to have negative margins on standard margin assessments.2
In the study, Dr. Smith noted unique attributes with the LumiSystem modality.
“pFGS (Pegulicianine fluorescence-guided surgery) is a cavity-based tool that identifies residual tumor within 2 to 5 mm from the surface of the lumpectomy cavity, rather than on the surface of the excised lumpectomy specimen, " wrote Dr. Smith and colleagues. "The pFGS cavity-based approach avoids the inherent problem of specimen-based approaches — correlating the location of tumor on an excised deformable specimen surface with the location of residual tumor in the breast cavity. pFGS also allows for repeat imaging of areas of concern during the initial operation to verify the removal of all positive signal areas."
References
1. Lumicell. Lumicell’s cutting-edge imaging platform receives historic FDA approval to illuminate residual breast cancer. Business Wire. Available at: https://www.businesswire.com/news/home/20240418089547/en/Lumicell%E2%80%99s-Cutting-Edge-Imaging-Platform-Receives-Historic-FDA-Approval-to-Illuminate-Residual-Breast-Cancer . Published April 18, 2024. Accessed April 18, 2024.
2. Smith BL, Hunt KK, Carr D, et al. Intraoperative fluorescence guidance for breast cancer lumpectomy surgery. NEJM Evid. 2023;2(7):EVIDoa2200333. doi: 10.1056/EVIDoa2200333.
How to Successfully Launch a CCTA Program at Your Hospital or Practice
June 11th 2025Emphasizing increasing recognition of the capability of coronary computed tomography angiography (CCTA) for the evaluation of acute and stable chest pain, this author defuses common misperceptions and reviews key considerations for implementation of a CCTA program.
Can Abbreviated MRI Have an Impact in Differentiating Intraductal Papilloma and Ductal Secretion?
June 3rd 2025For patients with inconclusive ultrasound results, abbreviated breast MRI offers comparable detection of intraductal papilloma as a full breast MRI protocol at significantly reduced times for scan acquisition and interpretation, according to a new study.
The Reading Room: Artificial Intelligence: What RSNA 2020 Offered, and What 2021 Could Bring
December 5th 2020Nina Kottler, M.D., chief medical officer of AI at Radiology Partners, discusses, during RSNA 2020, what new developments the annual meeting provided about these technologies, sessions to access, and what to expect in the coming year.