Cardiac Ultrasound
Latest News
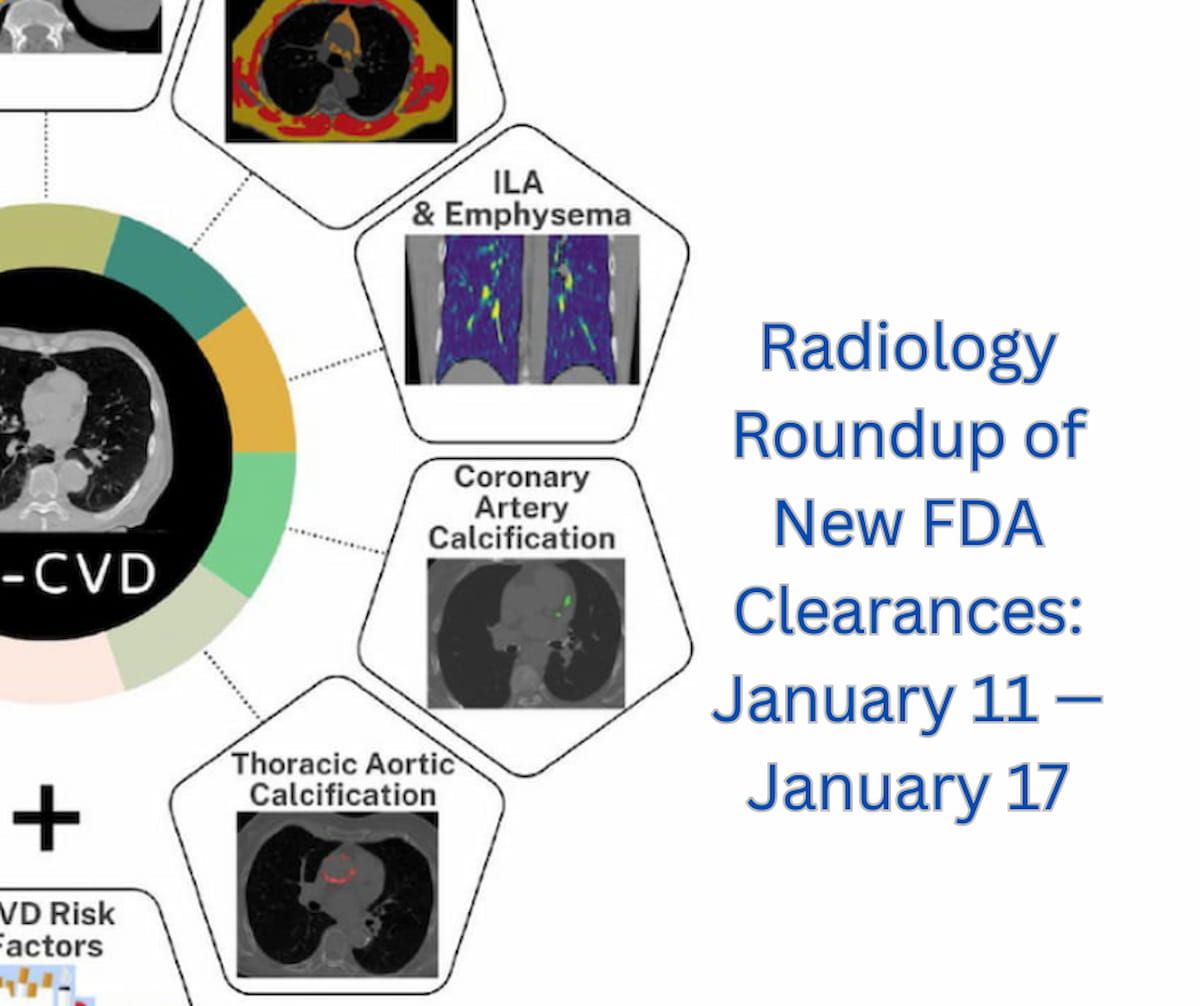
FDA Issues Expanded Clearance for UltraSight’s Cardiac Ultrasound Echo Stewardship Program
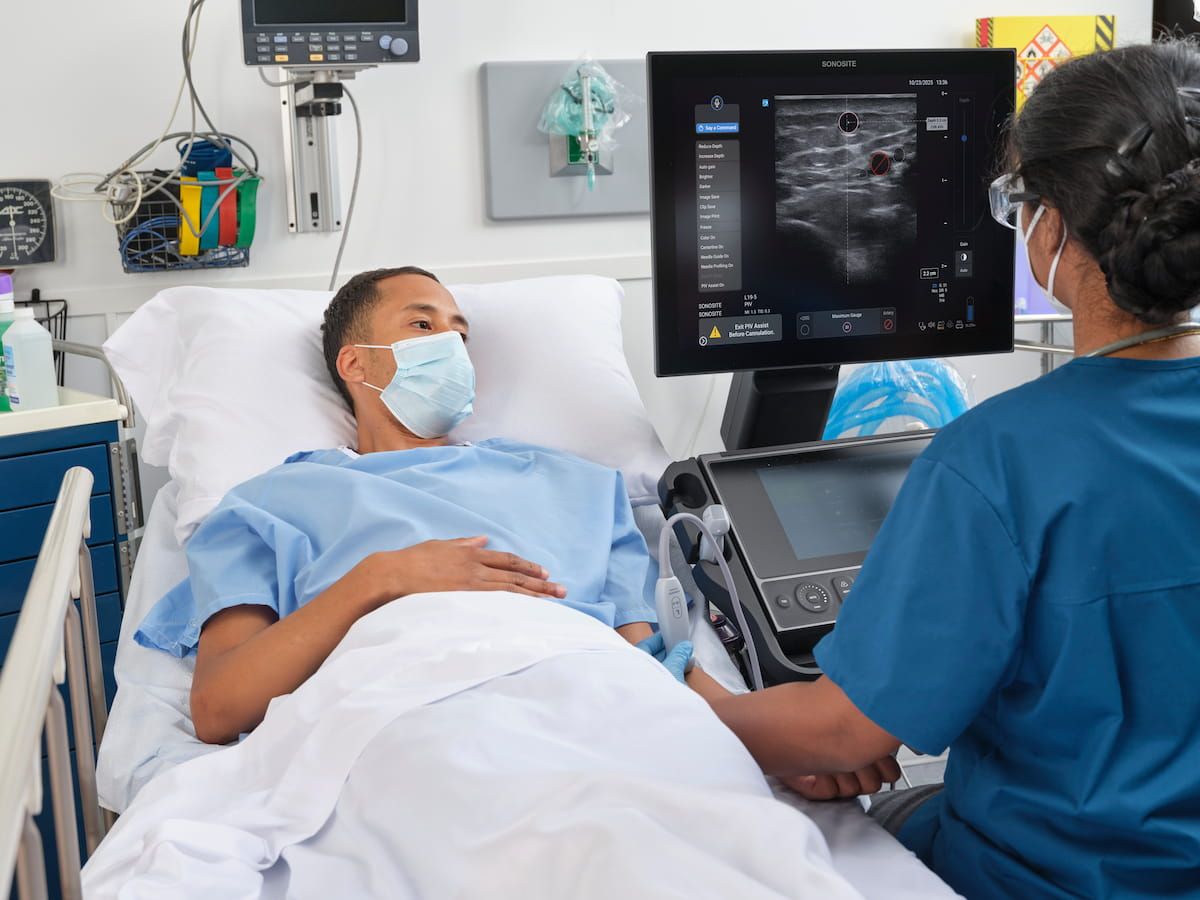
New AI-Powered Ultrasound Feature May Enhance Planning for Peripheral IV Procedures
Latest Videos

CME Content
More News
The Paradise Ultrasound Renal Denervation System has demonstrated efficacy and a favorable safety profile in randomized controlled trials for the treatment of mild, moderate and resistant hypertension.

Offering real-time, high-resolution ultrasound imaging for a range of procedures, the zero-capex Intracardiac Imaging System reportedly facilitates workflow efficiencies and reduced costs.

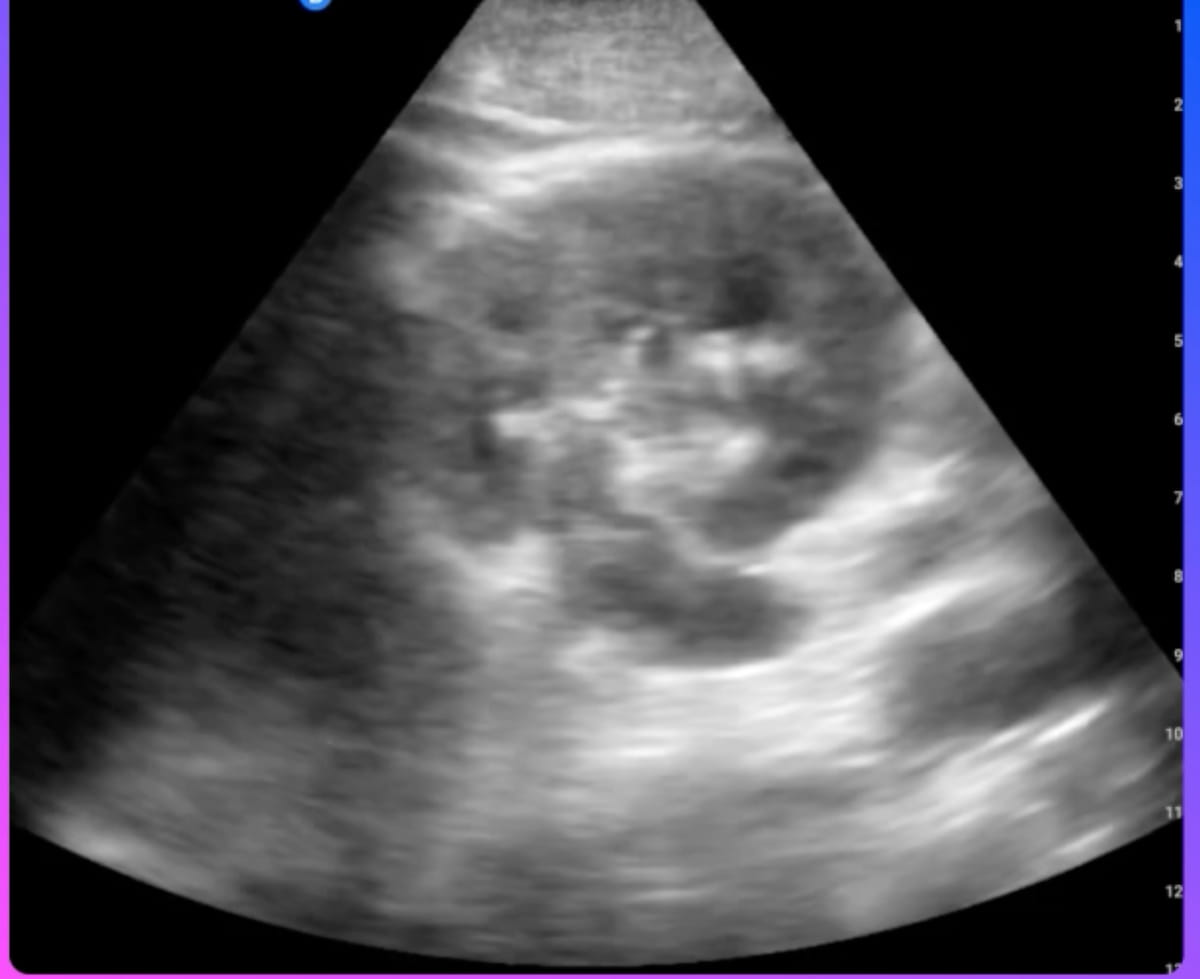
Designed for use in saline contrast echocardiography, the ASI-02 modality is currently being evaluated in a multicenter, randomized trial for patients undergoing transthoracic echocardiography (TTE).
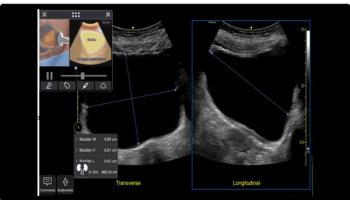
New enhancements for the Venue POCUS devices include automated labeling of anatomical landmarks with Nerveblox to facilitate 12 common peripheral nerve blocks and contrast-enhanced ultrasound geared to abdominal injury assessments.
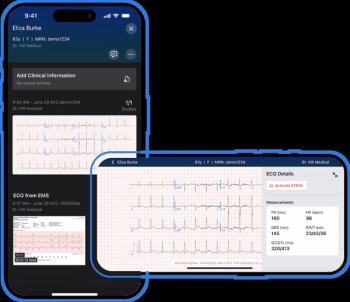
The AI-powered Viz ACS platform may facilitate improved communication between clinicians and more rapid treatment for cases involving acute coronary syndrome.
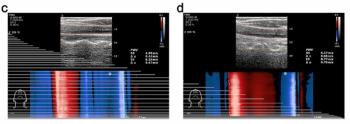
Findings from a multivariable analysis revealed that increased pulse wave velocity-end of systole (PWV-ES), assessed with ultrafast ultrasound, was associated with more than double the cardiovascular risk in young individuals with no major cardiovascular risk factors.
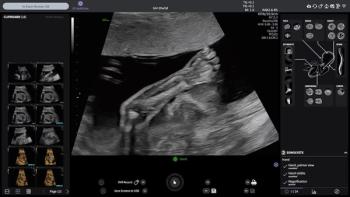
The Voluson Performance 18 and 16 ultrasound devices reportedly combine enhanced imaging capabilities with AI-enabled efficiencies.
The AI-powered cardiovascular ultrasound device reportedly offers enhanced spatial and contrast resolution as well as bolstered 4D imaging that enables improved evaluation of cardiac function for a wide range of patients.
Catch up on the most-well viewed radiology content in August 2025.
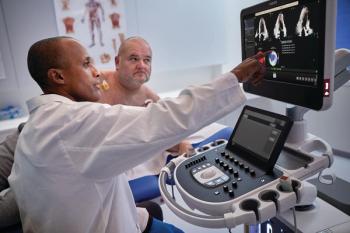
Combining advances in imaging quality with access to 26 FDA-cleared applications for automated and accelerated tasks, the Transcend Plus software will be featured at the upcoming European Society of Cardiology (ESC) and American Society of Echocardiography (ASE) conferences.

The MyLab A50 and MyLab A70 ultrasound platforms reportedly enable a variety of detailed and multiparametric evaluations, including assessments for liver elastography and strain analysis echocardiography.

Could the NeedleVue LC1 Ultrasound System reinvent real-time ultrasound-assisted needle guidance for interventional procedures?
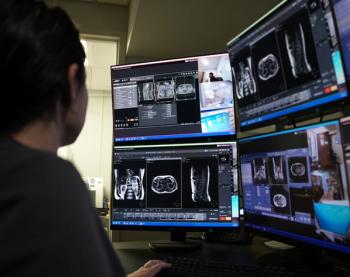
In addition to facilitating centralized scanning for a variety of imaging, the TechLive system may help ease the strain of technologist shortages and broaden access to advanced imaging exams.

Catch up on the top AI-related news and research in radiology over the past month.
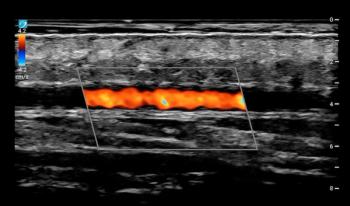
Offering a minimum scan depth of 4 mm, the UHF46-20 Transducer reportedly provides high-quality resolution for visualizing superficial nerves and vessels.

Stay updated with the latest in radiology, including PET, MRI, and AI research, plus essential insights on mammography and cardiac imaging.
EchoGo Amyloidosis, an echocardiography-based AI screening software, demonstrated a 93 percent AUROC for cardiac amyloidosis detection in a new multicenter study.
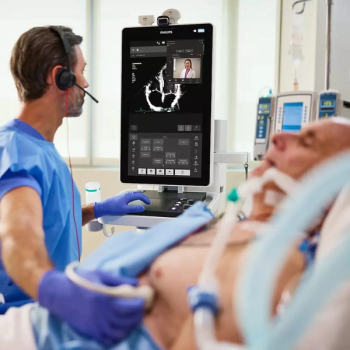
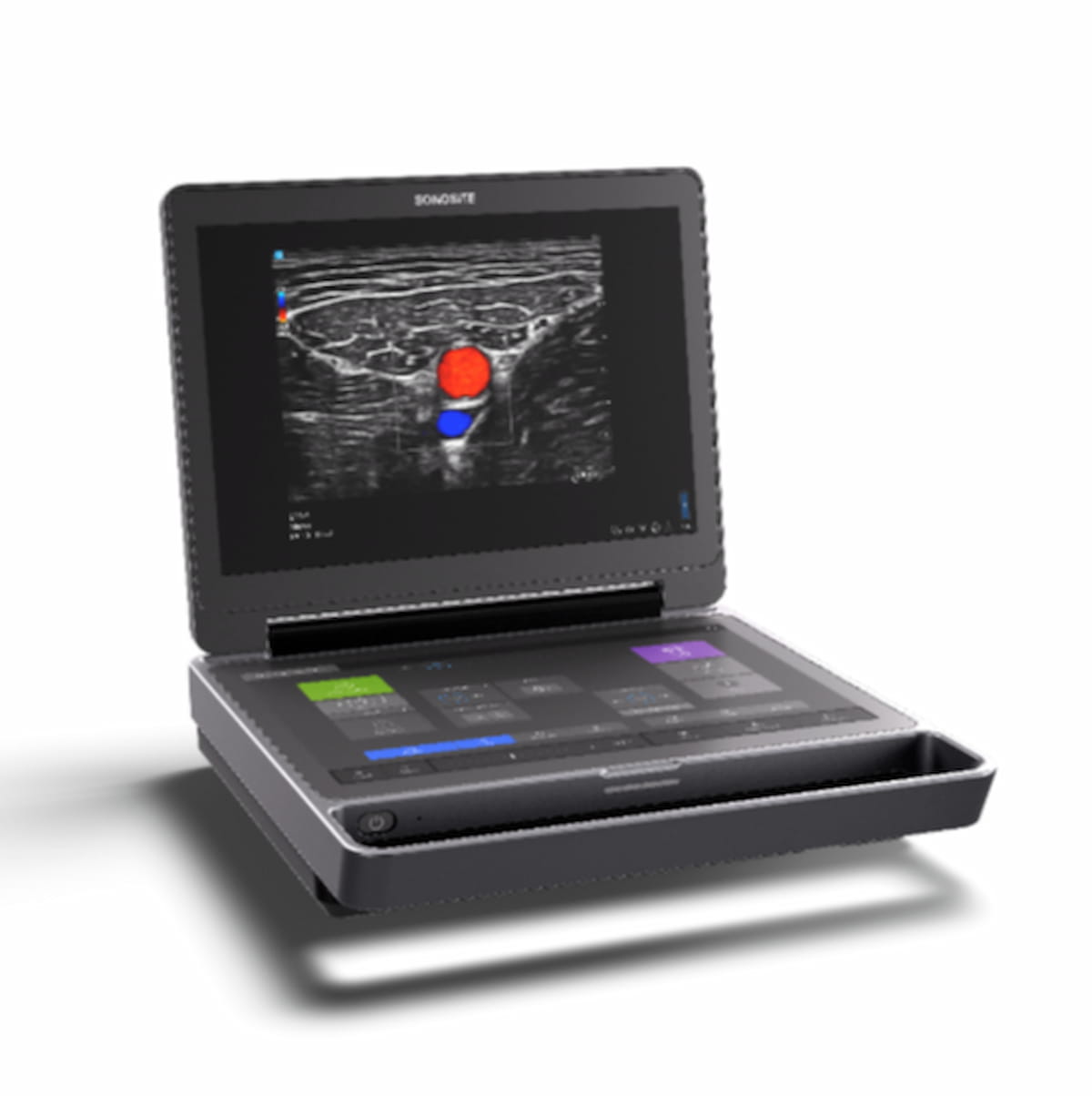
Offering a combination of intuitive touchscreen controls and enhanced image clarity, the portable Flash 5100 POC ultrasound platform reportedly facilitates confident and rapid assessment in emergency radiology and critical care settings.

Catch up on the top radiology content of the past week.
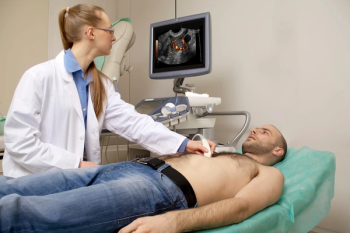
Those receiving ultrasound enhancing agents (UEAs) for transthoracic or stress echocardiography had lower odds of all-cause death in comparison to patients who did not have UEAs, according to a nationwide study involving 11.4 million patients.
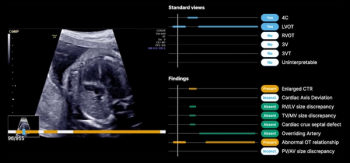
The latest FDA 510(k) clearance is for B-Right Views, an AI-enabled device, which provides automated detection of required views necessary for second- and third-trimester fetal heart ultrasound exams.

The only ultrasound enhancing agent that does not contain polyethylene glycol, Optison may now be utilized to enhance echocardiogram assessment of ventricular function in pediatric patients.
Catch up on the most-well viewed radiology content in April 2025.
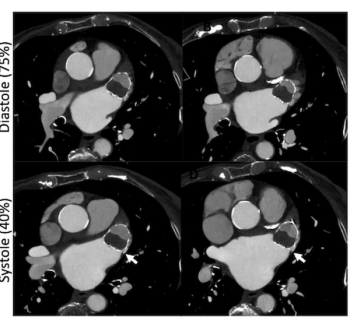
Cardiac CT angiography may provide insights on common post-op complications of left atrial appendage closure, ranging from peri-device leaks to device-related thrombus, according to research presented at the American Roentgen Ray Society (ARRS) conference.

Catch up on the top AI-related news and research in radiology over the past month.