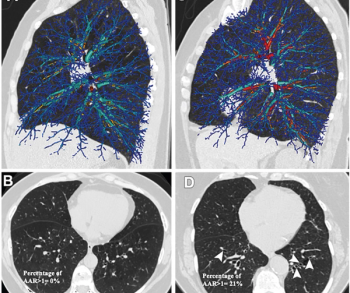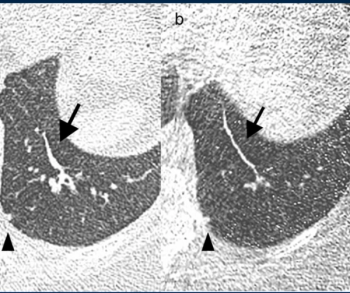
Reportedly the first targeted molecular imaging agent to provide intraoperative illumination of lung cancer, Cytalux (pafolacianine) helped identify clinically significant events in more than 50 percent of patients undergoing surgery for confirmed or suspected pulmonary nodules, according to Phase 3 trial data presented earlier this year at the American Association for Thoracic Surgery Annual Meeting.