Articles by Greg Freiherr

Dose reduction at one time played second fiddle to image quality, but today it enjoys top billing. New algorithms are coming into play in CT, radiography, and fluoroscopy to maintain image quality at traditional levels by processing out the noise that sneaks in during low-dose exams. In some cases, fear of radiation has gotten so bad that patients forego CT and other sources of ionizing radiation all together.
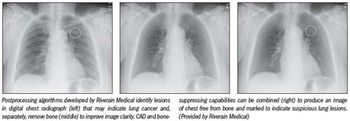
Bone can obscure lung lesions that may indicate cancer. The latest version of computer-assisted detection software from Riverain Medical, shown publicly for the first time in the RSNA 2010 exhibit hall, takes care of the problem.
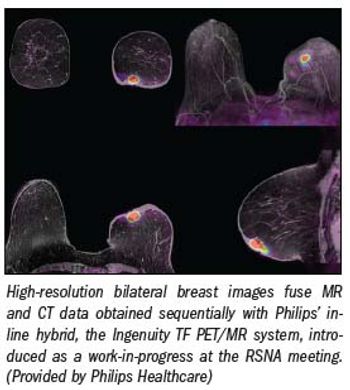
PET/MR is the logical next step in hybridization, the step that follows PET/CT, one that vendors finally took at this year's RSNA meeting. It has come a decade after PET/CT was first commercialized and nearly as long since pundits concluded that its arrival was inevitable.
Sonography aspires to pushbutton simplicity, but the nature of the technology stands in the way.
Demand for CT is dropping in some quarters of the imaging community, down by single-, sometimes even double-digit percentages.

Wireless x-ray detectors have come into vogue and are now available from a half dozen vendors. They are being offered singly, as a digital upgrade for analog x-ray systems, for example, or as the core of portable and advanced fixed radiography systems. GE Healthcare is among those offering a portfolio of such choices. But GE is putting a twist on its wireless detector, dubbed the FlashPad, one that company execs say will prevent what could be a ticklish problem in the future. This potential problem stems from the success of digital radiography.

A navigation system from Toshiba America Medical Systems has made complex interventions a little simpler.

Remote radiography and fluoroscopy systems traditionally have appealed more to Europeans than to practitioners in the U.S. But Philips is betting the time-and design of its new Juno DRF-are right to make a change in old habits. The Juno DRF remote-controlled flat-detector system, recently cleared by the FDA, offers enhancements that Philips Healthcare is promoting in its RSNA booth as the means to faster workflow and patient throughput and maximized return on investment.

Bone can obscure lung lesions that may indicate cancer. The latest version of CAD software from Riverain Medical, shown publicly for the first time at RSNA 2010, takes care of the problem.

Philips is adding to its portfolio a new line of CT scanners and a new line of PET/CTs based on dose-saving technologies. Ingenuity CT will displace Philips’ Brilliance line of CTs in configurations up to 128 slices; the Ingenuity TF PET/CT will displace the company’s Gemini platform of PET/CTs. Philips will not stop making these older models, but will feature them in its product portfolio as alternatives to the latest releases.

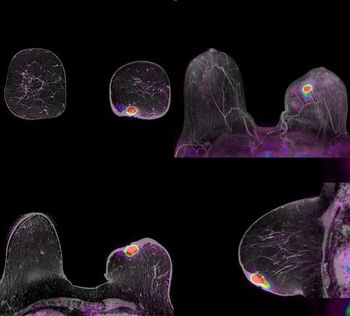
Hologic has pursued breast tomosynthesis as a commercial modality for the last five years, recruiting luminaries with track records in tomo going back a decade or more. Now, at RSNA 2010, the company is on the brink of achieving that objective.

A radiation monitoring system that quantifies the radiation being absorbed in the fluoro suite promises to give individual staff the information they need to minimize their exposure to x-rays. Philips Healthcare came up with the product, called DoseAware, which the company unveiled earlier this year at the European Congress of Radiology and now on the RSNA 2010 exhibit floor.

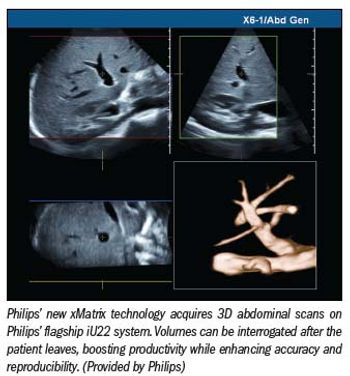
Philips’ new xMatrix brings real-time volumetric scanning to radiology as part of the company’s latest version of the iU22 ultrasound scanner. First built into Philips’ echocardiography systems five years ago, a souped-up version built into the company’s flagship iU22 on the RSNA exhibit floor quickly captures volumes in the abdomen that can be interrogated in 2D planes any time after the patient has left the exam room. Two planes can be viewed simultaneously using Live xPlane. Images drawn from the volume can then be sent to any PACS.

Walk into the lifeIMAGE booth on the RSNA 2010 exhibit floor and you will see how hundreds of thousands of patients will take control of their medical images and records over the next two years.

In the battle against patient overdose, Toshiba America Medical Systems brought two weapons to RSNA 2010. One is its Adaptive Iterative Dose Reduction (AIDR), software similar in approach to products from competing CT vendors who use iterative reconstruction to squeeze noise out of their images. The other, Target CTA, is a dose protocol devised specifically for cardiac scans done on the Aquilion One.

Fuji Medical Systems USA outdid itself in portable x-ray twice this year at the RSNA meeting. The company unveiled a wireless version of the cabled portable x-ray detector it released earlier in the year. It also brought out a new version of its FCR Go, a portable x-ray system based on computed radiography, featuring an enhanced generator, full-size workstation, and improved drive subsystem for greater mobility.

The effects of an agreement signed in the run-up to RSNA 2010 changed the IT approaches of two companies on the exhibit floor, TeraRecon and Agfa HealthCare. One week before the imaging community trekked to Chicago, these two companies cut a deal to make TeraRecon’s thin-client iNtuition an integral part of Agfa’s IMPAX 6 PAC system
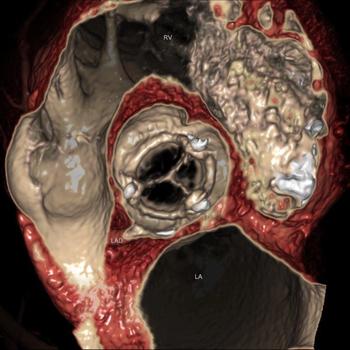
Nearly a decade after PET/CT was first commercialized, vendors at RSNA 2010 took the next logical step and combined MR and PET. The result is unlike anything seen before.

Fuji Medical USA has integrated 3D directly into its Synapse PACS, eliminating the need for thin-client 3D or dedicated 3D workstations accompanying its PACS. The newly integrated technology, dubbed Synapse 3D, was shown for the first time as a commercial product at RSNA 2010, accompanied by a work-in-progress application called Synapse Mobility that promises to allow access to Synapse PACS images and clinical tools using handheld devices.

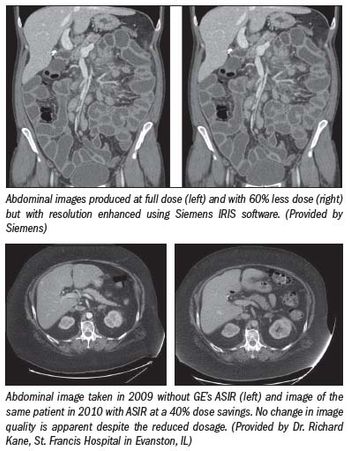
Seeking to drive down patient radiation dose, Siemens Healthcare unveiled a new technology platform for its CT scanners, one that not only cuts dose but speeds the exam and promotes more efficient patient handling
Video! Ziosoft has been trying to unlock the U.S. market for advanced visualization for several years. It may have found the key with a technology unveiled this week at RSNA2010.

Video! Diagnostic Imaging's Greg Freiherr interviews representatives of the major vendors exhibiting at this year's meeting.

Hologic is on the brink of realizing its ambition to pioneer the commercial use of 3D mammography in the U.S. The company, which for 25 years has focused on women’s health, announced receipt of an approvable letter from the FDA for its Selenia Dimensions digital mammography tomosynthesis system.

When the gatekeepers clear the way to the exhibit halls at McCormick Place, Canon USA will be out with its CXDI-70C Wireless Digital Radiography System, but it won’t be alone. A gaggle of vendors, including Carestream and Fuji, will be showing portable x-ray detectors as well.

The need for increased efficiency will continue to ripple across radiology, as it has since the start of the great recession, leading the community to seek better and lower cost ways to manage patients. This year, as in years past, this need will be satisfied in large part by offerings in information technology.

Protocols that get the most from every x-ray and high-tech algorithms that delete noise will drive CT at this year’s show.

MR vendors will show advanced technologies in traditional-and nontraditional-realms at the RSNA meeting.

The technological and political evolution of digital mammography will be in evidence on the RSNA exhibit floor.

Teleradiology provider Radisphere is targeting community hospitals unhappy with small radiology group practices.

Vendors have tried to walk a thin line between providing equipment and prescribing its use. Automated protocols and image processing have been enhanced to make imaging products easier to use and the images they deliver more consistent from one user to another. But they have stopped short of mandating technologies that otherwise impact the practice of medicine. Now the FDA wants equipment makers to expand their role.






