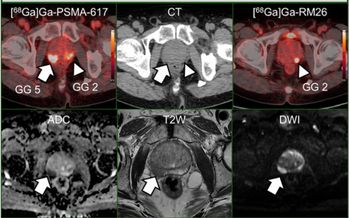
External fixation devices and MRI safety
Most orthopedic implants and materials do not pose problems for patients undergoing MRI procedures. MRI may be hazardous for external fixation systems, however, because of the length of the implant or the formation of a conductive loop.
Frank Shellock, Ph.D.
Adjunct clinical professor of radiology and medicine, University of Southern California and Institute for Magnetic Resonance Safety, Education, and Research
www.MRIsafety.com
www.IMRSER.org
Most orthopedic implants and materials do not pose problems for patients undergoing MRI procedures. MRI may be hazardous for external fixation systems, however, because of the length of the implant or the formation of a conductive loop.
External fixation systems are specially designed frames, clamps, rods, rod-to-rod couplings, pins, posts, fasteners, wire fixations, fixation bolts, washers, nuts, hinges, sockets, connecting bars, screws, and other components used in orthopedic and reconstructive surgery.
Indications for external fixation systems are varied, including the following treatment applications:
- open and closed fracture fixation
- pseudoarthroses of long bones (congenital and acquired)
- limb lengthening by metaphyseal or epiphyseal distraction
- correction of bony or soft-tissue defects
- correction of bony or soft-tissue deformities
The assessment of MRI safety issues for external fixation systems is especially challenging because of the myriad possible components, many of which are made from conductive materials, and the many configurations used for these devices.
The primary concern is MRI-related heating, which is dependent on the particular aspects of the external fixation system. The specific MRI conditions - strength of the static magnetic field, radiofrequency, type of RF transmit coil, pulse sequence, body part imaged - directly affect the safety aspects of scanning patients with external fixation systems.
For each external fixation device that has undergone MRI safety testing and applied for approval of labeling from the FDA, highly specific guidelines need to be followed relative to a given device configuration and the MRI conditions used for the imaging procedure. This information will be contained in the Instructions for Use for a given external fixation device. Imaging protocols will vary accordingly.
REFERENCE
Shellock FG. Reference manual for magnetic resonance safety, implants, and devices. 2006 ed.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.