Women's Health Ultrasound
Latest News
Latest Videos

Shorts

CME Content
More News
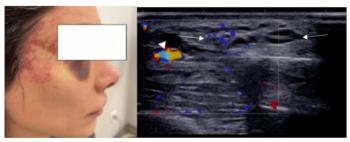
In a new literature review, researchers discuss common patterns and complications with cosmetic fillers that can be revealed on ultrasound imaging.

Catch up on the most well-viewed radiology content from 2025.
Catch up on the most well-viewed ultrasound content from 2025.
The recently launched PIV Assist reportedly offers a variety of features including real-time calculation of the catheter-to-vein ratio and pre-scan differentiation of veins and arteries to facilitate vascular access planning.
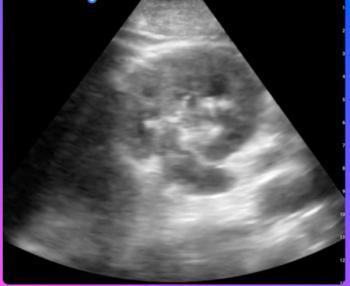
The newly launched Compass AI software platform reportedly facilitates a 94 percent compliance rate for documentation of point-of-care ultrasound (POCUS) exams.
The Sonosite MT ultrasound device reportedly combines portability and enhanced high-resolution images with multiple features to promote workflow efficiency.
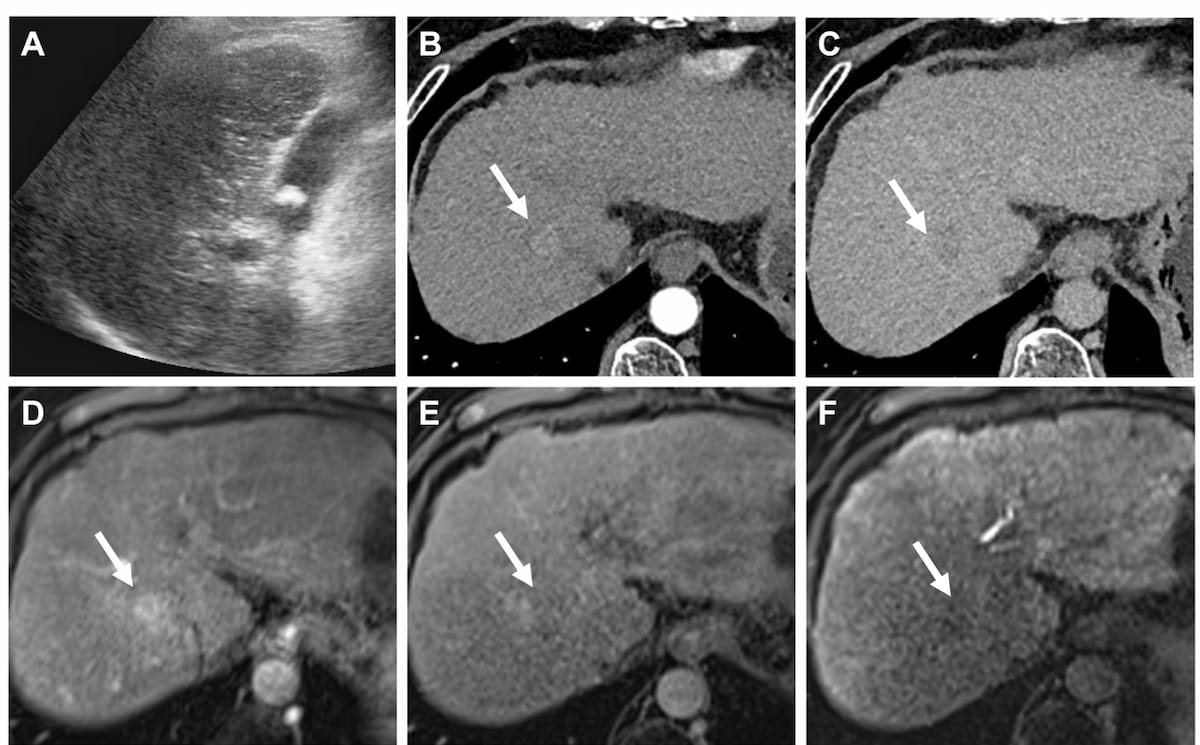
Ultrasound-derived fat fraction assessment offered higher AUCs for differentiating mild, moderate and severe hepatic steatosis in comparison to established noninvasive measures of metabolic dysfunction-associated steatosis liver disease (MASLD), according to new multicenter research.
Already in use in multiple countries, the Pulsenmore ES ultrasound platform reportedly enables expectant mothers to transmit ultrasound video clips through a smartphone app for remote interpretation.
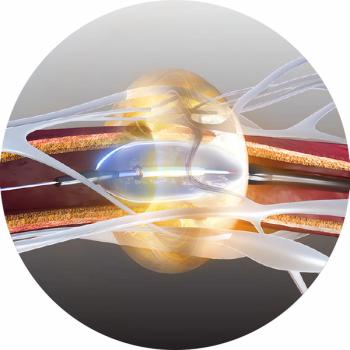
The Paradise Ultrasound Renal Denervation System has demonstrated efficacy and a favorable safety profile in randomized controlled trials for the treatment of mild, moderate and resistant hypertension.

Offering real-time, high-resolution ultrasound imaging for a range of procedures, the zero-capex Intracardiac Imaging System reportedly facilitates workflow efficiencies and reduced costs.

Designed for use in saline contrast echocardiography, the ASI-02 modality is currently being evaluated in a multicenter, randomized trial for patients undergoing transthoracic echocardiography (TTE).
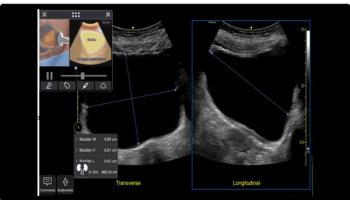
New enhancements for the Venue POCUS devices include automated labeling of anatomical landmarks with Nerveblox to facilitate 12 common peripheral nerve blocks and contrast-enhanced ultrasound geared to abdominal injury assessments.
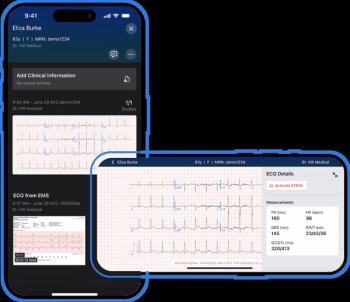
The AI-powered Viz ACS platform may facilitate improved communication between clinicians and more rapid treatment for cases involving acute coronary syndrome.
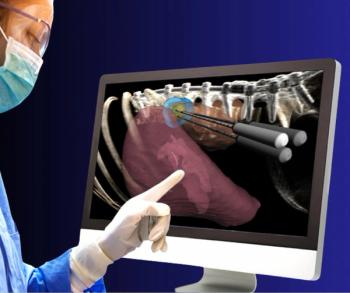
Previously cleared for liver tumor ablation therapy, the BioTraceIO360 ultrasound software reportedly bolsters pre-procedure planning with ablation zones.
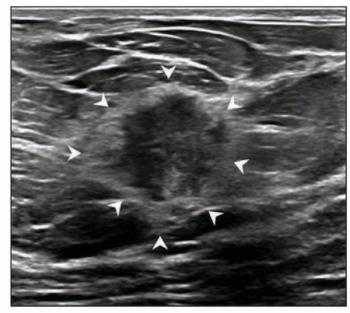
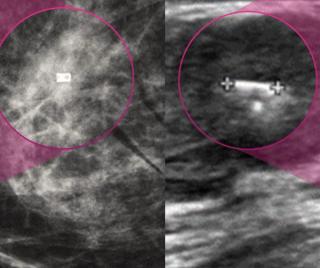
For masses interpreted as BI-RADS category 4 and 5 presentations on breast ultrasound, the authors of a new study found that the presence of echogenic rind had an 81 to 85 percent specificity for malignancy.
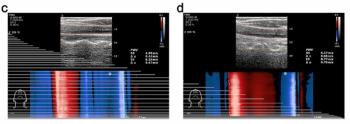
Findings from a multivariable analysis revealed that increased pulse wave velocity-end of systole (PWV-ES), assessed with ultrafast ultrasound, was associated with more than double the cardiovascular risk in young individuals with no major cardiovascular risk factors.
The Voluson Performance 18 and 16 ultrasound devices reportedly combine enhanced imaging capabilities with AI-enabled efficiencies.
The AI-powered cardiovascular ultrasound device reportedly offers enhanced spatial and contrast resolution as well as bolstered 4D imaging that enables improved evaluation of cardiac function for a wide range of patients.
Catch up on the most-well viewed radiology content in August 2025.
Combining advances in imaging quality with access to 26 FDA-cleared applications for automated and accelerated tasks, the Transcend Plus software will be featured at the upcoming European Society of Cardiology (ESC) and American Society of Echocardiography (ASE) conferences.

Catch up on the top AI-related news and research in radiology over the past month.

The MyLab A50 and MyLab A70 ultrasound platforms reportedly enable a variety of detailed and multiparametric evaluations, including assessments for liver elastography and strain analysis echocardiography.

Catch up on the top radiology content of the past week.
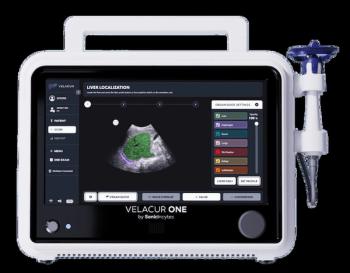
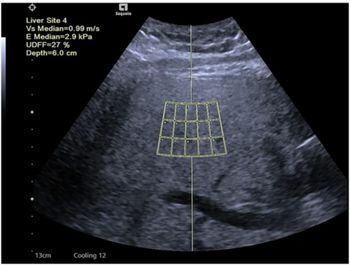
Reportedly the only point-of-care ultrasound system that can estimate liver stiffness and attenuation that correlate to MRI-PDFF, Velacur One also may facilitate higher reimbursement than non-imaging elastography.

Could the NeedleVue LC1 Ultrasound System reinvent real-time ultrasound-assisted needle guidance for interventional procedures?