Imaging Findings May Help Detect CAR T-Cell Therapy Toxicities
Study findings further highlight the central role of PET/CT in disease response after CAR-T cell therapy.
In patients with refractory B-cell lymphoma, thoracic imaging findings correlated with cytokine release syndrome grade following chimeric antigen receptor (CAR) T-cell infusion. This is according to a study published in
“Imaging plays a critical role in the evaluation of clinical cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome (ICANS) toxicity following CAR T-cell treatment,” wrote Daniel Smith, M.D., a radiologist at the University Hospitals Cleveland Medical Center in Ohio. “Specific findings at thoracic imaging—in particular, pleural effusion and atelectasis—may prove useful correlates for severe clinical grades of cytokine release syndrome, while findings related to ICANS tend to be less specific and less frequent. “
CAR T-cell immunotherapy is increasingly used for refractory lymphoma, but it may lead to cytokine release syndrome and ICANS in the early period following infusion. The associations between cytokine release syndrome or ICANS grade and imaging findings are not established. The goal of this study was to characterize thoracic and neuroimaging findings associated with cytokine release syndrome and ICANS in patients with refractory B-cell lymphoma.
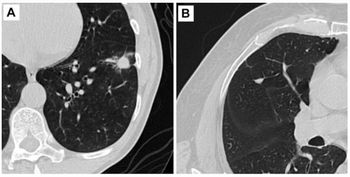
The retrospective analysis included 38 patients (mean age 59; 61% men) with refractory B-cell lymphoma who underwent CAR T-cell infusion. Of these, 63% and 29% experienced clinical grade 1 or higher cytokine release syndrome and ICANS, respectively. Patients with grade 2 or higher cytokine release syndrome were more likely to have thoracic imaging evidence — at radiography, CT and/or MRI — of pleural effusions (36% vs 8.3%, P = .04) and atelectasis (57% vs 25%, P = .048) than patients without cytokine release syndrome or with grade 1 toxicity. Positive imaging findings were identified in 43% with grade 2 or higher ICANS who underwent neuroimaging. Patients undergoing CAR T-cell therapy had higher rates of clinical response, including 56% with complete response and 19%with partial response.
“Our findings further highlight the central role of PET/CT in disease response after CAR-T cell therapy,” the authors wrote.
Limitations of the study included its retrospective design, small sample size, and that most patients without toxicity did not undergo imaging.
In an accompanying
“As more data are prospectively obtained, imaging guidelines to better monitor for the important conditions examined here may be established, and the information collected used to effectively intervene early in detection and treatment,” Dr. Langer wrote.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.