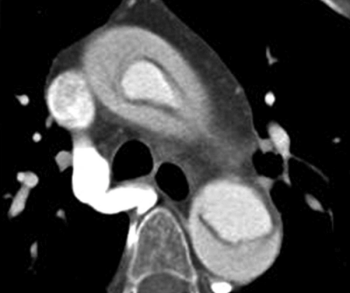
New research suggests the Viz Aortic Dissection Algorithm has a 94.2 percent sensitivity rate for detecting aortic dissection on computed tomography (CT) angiography.

New research suggests the Viz Aortic Dissection Algorithm has a 94.2 percent sensitivity rate for detecting aortic dissection on computed tomography (CT) angiography.

Catch up on the top radiology content of the past week.
In a recently published article, researchers from Yale University discuss the pros and cons of current FDA regulations as they apply to the clearance and use of adjunctive artificial intelligence (AI) software with conventional breast cancer screening modalities such as mammography.
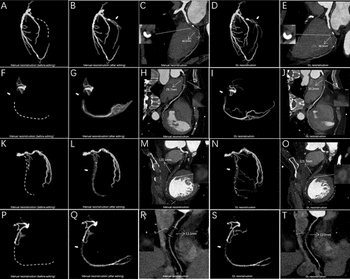
Emerging research revealed that a deep learning model had a nearly twofold increase in successful segmentation and reconstruction of coronary total occlusions (CTOs) on coronary computed tomography angiogram (CCTA) and a 73 percent reduction in post-processing and measurement time in comparison to a conventional manual approach.

Catch up on the top radiology content of the past week.
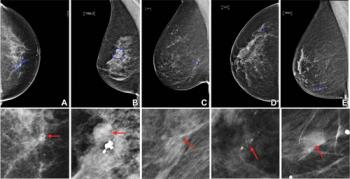
In separate test sets of Israeli women and United States women who had either ductal carcinoma in situ or invasive breast cancer, emerging artificial intelligence (AI) algorithms achieved an area under the curve (AOC) of 88 percent and 80 percent, respectively, for malignancy detection.

Catch up on the top AI-related radiology content of the past month.

Catch up on the top radiology content of the past week.
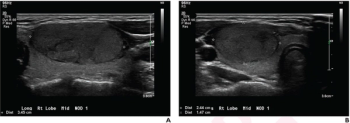
The retrospective study of patients 21 years of age or younger found that a deep learning algorithm and use of the American College of Radiology’s Thyroid Imaging Reporting and Data System (TI-RADS) both had more than a 26 percent greater sensitivity for differentiating thyroid nodules on ultrasound in comparison to radiologist assessment.
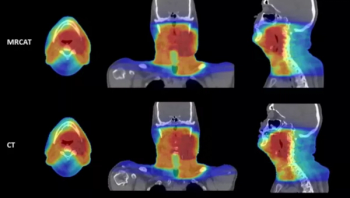
For physicians performing radiotherapy treatment of soft tissue tumors in the head and neck, the MRCAT Head and Neck offers an artificial intelligence (AI) application that allows the use of magnetic resonance imaging (MRI) as the primary or sole imaging for procedure planning.
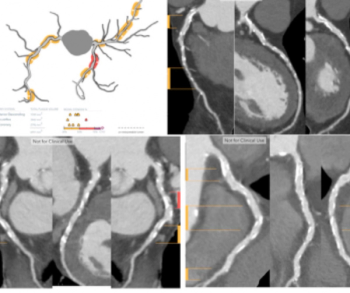
Plaque Analysis and RoadMap Analysis, two artificial intelligence (AI)-enabled assessment products, may enhance clinical evaluation of coronary artery disease (CAD) on cardiac computed tomography angiography (CCTA).

Catch up on the top radiology content of the past week.
While artificial intelligence (AI) models have been acknowledged for aiding imaging analysis or facilitating workflow enhancements, this author envisions AI as a potential workstation conceierge capable of turning common venting and gripes into actionable items for significant improvements.
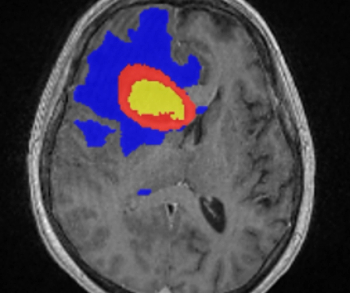
Incorporating artificial intelligence (AI)-based technology, Neosoma HGG reportedly demonstrated a 95.5 percent accuracy rate in measuring brain tumor volume on brain magnetic resonance imaging (MRI) scans at various points during the treatment of patients with high-grade gliomas.

TeraRecon Neuro reportedly offers six automated and customizable computed tomography (CT) perfusion maps that facilitate assessment of brain function in hemorrhagic and ischemic neurological cases.
Addressing upgrades of traditional infrastructure used in everyday radiology practice may be a more practical use of resources than investment in artificial intelligence (AI) technology that is still evolving.
AIR Recon DL, a deep learning-based image reconstruction software, will now be available with 3D sequences as well as PROPELLER motion-insensitive sequences on magnetic resonance imaging (MRI) scanners from GE Healthcare.

Catch up on the top AI-related radiology content of the past month.
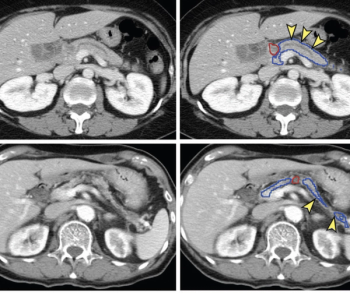
New research reveals that an emerging deep learning tool had comparable sensitivity and specificity to radiologist assessment of contrast-enhanced computed tomography (CT) scans for pancreatic cancer, and a 74.7 percent sensitivity rate for tumors smaller than 2 cm.
Assessing high-resolution computed tomography (CT) scans of the wrist, CurveBeam AI’s OssView software reportedly enables clinicians to ascertain bone fragility and fracture risk for women over the age of 70.

Catch up on the top radiology content of the past week.
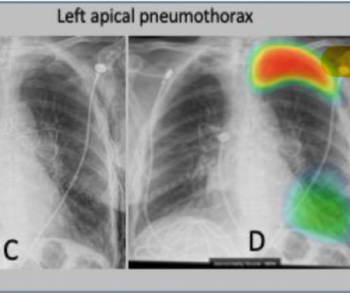
Researchers found that stand-alone use of an artificial intelligence (AI) model led to a 24.9 percent increase in sensitivity for diagnosing pulmonary nodules and a 21.4 percent increase in sensitivity for diagnosing pneumonia.