
Magnetic resonance imaging can help quantify the loss of gray matter in childhood-onset schizophrenia, bipolar disorder and other psychoses, possibly helping diagnose the cases to become more serious.

Magnetic resonance imaging can help quantify the loss of gray matter in childhood-onset schizophrenia, bipolar disorder and other psychoses, possibly helping diagnose the cases to become more serious.

The positron emission tomography tracer C-deuterium-L-deprenyl (11C-DED) can help physicians visualize astrocytes, which is thought to conspire with amyloid-beta plaques in the progression of Alzheimer disease.

Endovascular lower-extremity revascularization procedures performed by interventional radiologists bring about less transfusion and intensive-care unit use, shorter hospital stays, fewer repeat revascularization procedures, fewer amputations, and lower costs compared to those done by vascular surgeons.

The use of clinical decision support in the emergency room cuts the need for CT pulmonary angiography for acute pulmonary embolism by 20 percent and boosts yield 69 percent, according to a study published online on Dec. 20 in the journal Radiology.
The U.S. Food and Drug Administration has cleared the new version of MIM Software’s mobile imaging app, which is now approved for diagnostic X-ray and ultrasound viewing, the company announced.

The U.S. Food and Drug Administration has approved the first handheld device to detect intracranial hematomas - bleeding in the skull - which the agency says can help determine if patients with critical injuries need an immediate CT scan.

CMS won't apply 25 percent professional component Multiple Procedure Payment Reduction in 2012 for same practice-different physician services.
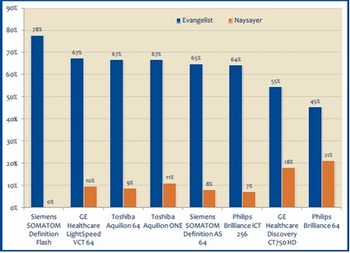
While providers are paying special mind in lowering radiation dose through prep work and process improvements, they do have their opinions on which CT vendors are the low dose leaders, according to a new report from KLAS, CT 2011: Focused on Dose.

University of Pennsylvania researchers have developed an experimental MRI contrast agent capable of targeting tumors, according to a report in the journal ACS Nano. Its coating, instead of targeting particular cancer receptors - which can be hit-or-miss and depend on the cancer - is attracted to the acidic environments in which tumors generally thrive.

Magnetic resonance imaging does not appear to help patients slated for epidural steroid injections (ESI) for chronic lower back pain, and has only a minor effect on the physician’s decision making, according to a study published online this week by the Archives of Internal Medicine.
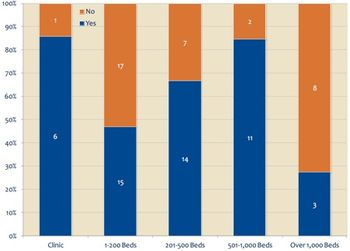
While 71 percent of healthcare organizations are either using or considering the deployment of cloud computing or storage technologies, trust in public-cloud services such as those offered by Amazon, Google and others remains weak, according to a new KLAS report. PACS is an area of particular interest in terms of leveraging the cloud, according to the report.

Patient familiarity with a medical imaging test makes them more likely move forward with the test, according to a survey of U.S. adult attitudes toward imaging released at RSNA this week.

CHICAGO - Breast-Specific Gamma Imaging (BSGI) has greater sensitivity and comparable specificity compared to mammography and ultrasound, according to a new study presented Tuesday at RSNA. A second study presented Tuesday showed that, unlike mammography, BSGI is as effective in detecting breast cancer in women with dense and non-dense breasts.

Magnetization transfer (MT) contrast-prepared magnetic resonance imaging is “unlikely to be of clinical utility” in diagnosing cirrhosis, according to a study published online Nov. 23 in the journal Radiology.

Although a vast majority of radiologists qualify under the federal government’s meaningful use incentive program for electronic health records, many have lingering concerns about the program, according to a new KLAS and RSNA survey.

Siemens’ computed tomography (CT) iterative reconstruction protocol has been cleared by the FDA, the company announced Wednesday.

Canon USA Inc. has received FDA 510(k) clearance for its digital radiography detectors, the company announced Tuesday.

Medicare spending on medical imaging continues to decline and that Medicare patients are receiving fewer imaging procedures, according to a new analysis of Medicare data released Nov. 17 by the Medical Imaging & Technology Alliance (MITA).

Radiologists order just 5.3 percent of high-cost imaging examinations, according to a study published online on Nov. 14 in the journal Radiology.

Coronary artery calcification (CAC) scores of zero don’t give patients a pass from obstructive coronary artery disease, according to a study using coronary computed tomography angiography (CCTA) on patients with symptoms of coronary artery disease.
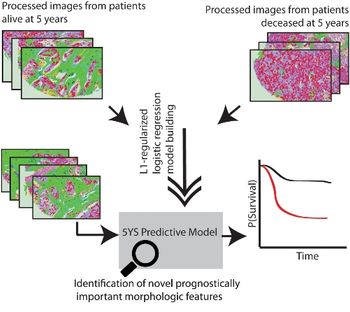
A team of Stanford engineers and pathologists have trained computers to analyze breast-cancer pathology slides - and the machine is more accurate than the assessments of man (and woman), the researchers said.

Medical imaging managers reported low confidence that reimbursement rates will improve and that funding for capital purchase for equipment and IT will be available in the fourth quarter of 2011 (October to December).

Spinal bleeding is a hallmark for child abuse, so complete spine imaging should be performed for young children undergoing brain magnetic resonance imaging for moderate or severe traumatic brain injury, according to a study published online Nov. 8 in the journal Radiology.

Inhaling undiluted, hyperpolarized xenon 129 for magnetic resonance imaging of the lungs is safe for chronic obstructive pulmonary disease (COPD) patients as well as healthy volunteers, Duke University researchers reported online on Nov. 4 in the journal Radiology.

The Centers for Medicare and Medicaid Services’ 25-percent cut of multiple procedure payment for image interpretation is “unfounded and potentially dangerous,” the American College of Radiology said.

Agreement is lacking - both across institutions and within departments - for the management of six commonly encountered incidental findings on body CT, concludes a study in the November issue of the Journal of the American College of Radiology. Departments should develop guidelines to ensure consistent patient recommendations, authors said.

It’s a question posed in a The New York Times article last week that explored MRIs in sports medicine.

New custom-designed patient shielding devices should supplant traditional lead aprons for chest CT scans - and possibly every scan, regardless of body part. That’s according to the authors of a new study published in the British Journal of Radiology.

Annual chest X-ray screenings have no effect on lung-cancer mortality, according to a study published online Oct. 26 in the Journal of the American Medical Association.

Nonradiologist physicians with a financial interest in imaging means were as much as 49 percent more likely to order imaging as those with no financial interest, according to a new study published in the American Journal of Roentgenology.