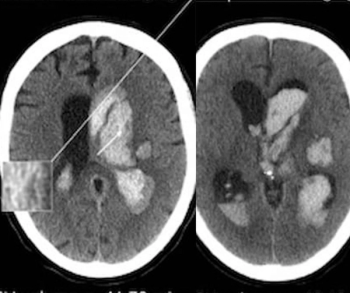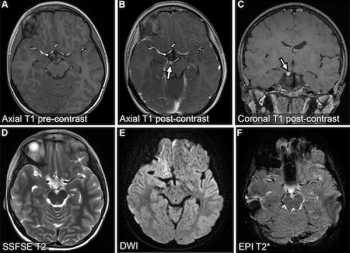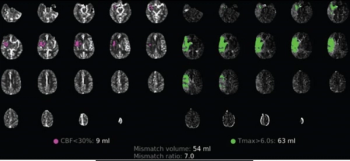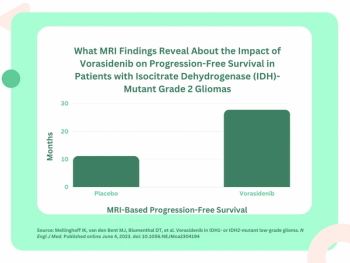
For patients with residual or recurrent grade 2 isocitrate dehydrogenase (IDH)-mutant gliomas, magnetic resonance imaging (MRI) revealed that daily dosing of vorasidenib, an inhibitor of mutant IDH1 and IDH2 enzymes, led to a median progression-free survival of 27.7 months in comparison to 11.1 months in a placebo group, according to new research presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago.