MRI may help physicians identify patients with Parkinson’s disease who may develop visual hallucinations.

MRI may help physicians identify patients with Parkinson’s disease who may develop visual hallucinations.

Case History: 40-year-old female presented with history of epigastric fullness associated with epigastric and left flank pain and burning micturition.

MRI detects brain serotonin levels in patients with mild cognitive decline from Alzheimer’s disease.
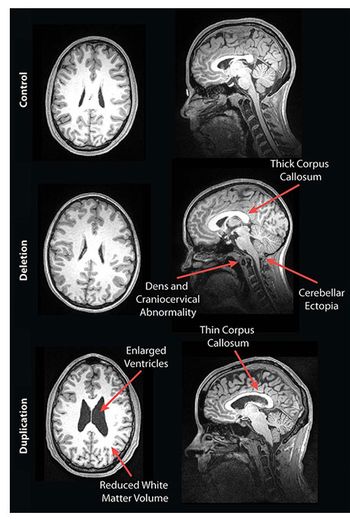
MRI has shown structural abnormalities related to cognitive function among people with genetic forms of autism.

Combined MRI can help physicians track cognitive impairment among professional fighters.
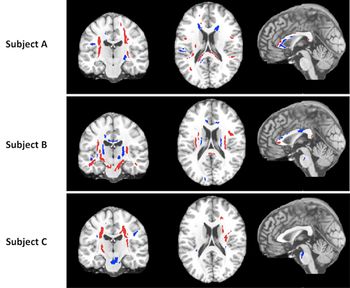
MRI may help physicians monitor Parkinson’s disease progression.
Review of MRI in the emergency department compared rates of MS as differential diagnosis and final diagnosis.

Case History: 28-year-old male presents with history of headaches.

Radiation exposure does not appear to be associated with malignant intracranial tumors among radiologic technologists.

Functional MRI may help determine which patients with depression will respond to antidepressant therapy.

Diffusion tensor imaging may help diagnose vascular cognitive disorders among patients with CAD.
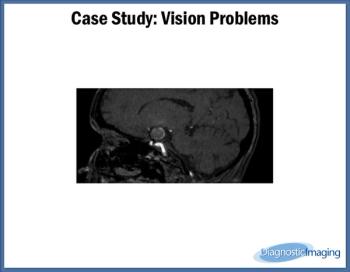
Case History: 67-year-old female presented with one week of vision problems.
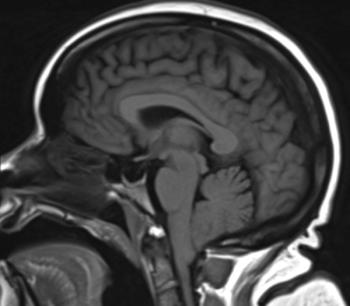
52-year-old female with vertigo, headache, tinnitus, eye twitching, and general instability while walking.
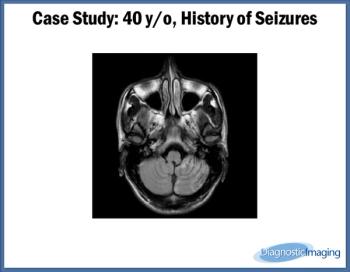
Case History: 40-year-old patient presented with history of seizures.

Images show that people with Parkinson’s disease and cognitive impairment have brain network disruptions.

Case History: 45-year-old male with history of alcoholism with history of four seizures one day earlier.

MRI shows exercise may help adults with mild cognitive impairment.
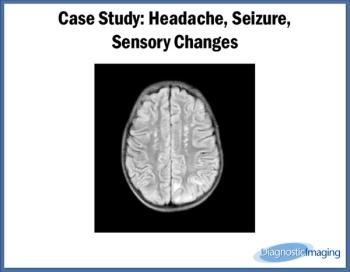
Case History: 32-year-old patient presented with complaint of headache, seizures, and sensory changes.

MRI indicates that traumatized boys and girls may have differences in insula subdivision structure.

Future neuroimaging studies of psychotherapy response may focus further on individual regions in the brain.

MRI images often detect incidental findings in older patients.

MRI highlights changes in brains of children with Tourette syndrome.

MRI shows brain changes in children with PTSD.

MRI shows brain changes in children who play football even if not diagnosed with concussion.
Case History: Four-year-old patient with complaints of altered sensorium.